Neurons have a distinct shape. The long processes for sending a signal (the axon) and for receiving signals (dendrites) are often far away from the cell body (soma) containing the nucleus. Thus, neurons have sophisticated systems for transporting messenger RNAs (mRNAs) and proteins involved in protein synthesis to these cellular processes for locally regulated protein translation. The tip of a developing axon is a specialized structure called a growth cone. Growth cones receive signals from the surrounding cells to navigate to their target. Once at the target, the growth cone will become the presynaptic side of the neuronal connection called a synapse. In the brain, axons make synapses with other neurons on dendrites. In the periphery, neurons form synaptic connections with various tissues and organs, such as the neuromuscular junction where the motor neuron innervates the fibers of a skeletal muscle. Other peripheral neurons, such as sensory neurons, form synapses in the spinal cord and receive input from the peripheral tissues. By understanding what proteins are present in axons and growth cones, how the proteins in the processes change through new protein synthesis, and how this local protein synthesis is regulated, researchers may discover ways to help regenerate neurons lost through injury or disease.
A kinase called mTOR is a critical regulator of protein translation in all cells. A pair of articles finds a key role for a master regulator of protein synthesis in injured axons and in growth cones. Terenzio and colleagues studied mice with injured sciatic nerves. The cell body of the sciatic nerve is located in the dorsal root ganglia near the spinal cord (Figure 1). The axon of the sciatic nerve extends in two directions: One part goes to down the leg to the periphery and the other goes to the spinal cord. Thus, this system is well suited for studying differences in protein content, protein synthesis, and the response to injury, because the cell body can be easily separated from the part of the axon in the periphery and the part of the axon in the spinal cord.
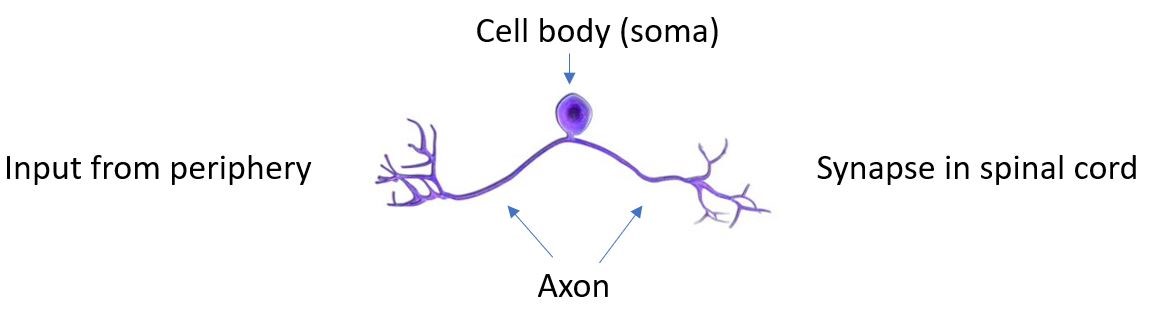
Figure 1. Morphology of the sciatic nerve. [More details]
Terenzio and colleagues studies local protein translation and its regulation in response to injury to the part of the axon in the periphery. They found that axon damage stimulated the delivery of the mRNA for mTOR into the injured region, where it was locally translated and stimulated local translation of various proteins. Injection of an inhibitor of mTOR into the axon prior to injury impaired axon outgrowth and reduced survival of these sensory nerves. Using this inhibitor, they also performed a proteomic analysis, which identified ~550 proteins, the “mTOR-dependent translatome.” Many transcripts of the mTOR-dependent translatome encoded proteins involved in promoting survival and mediating the response to axonal injury. Some of the newly translated proteins represented signals produced at the site of the injury that were sent back to the cell body to tell the nerve about the injury and initiate changes in gene expression in the nucleus. Thus, the nerve senses the damage, which triggers the expression of the mTOR gene and delivery of the mTOR mRNA to the site of injury, where mTOR is translated and triggers the production of proteins that send messages back to the nucleus, as well as proteins that help the axon recover at the site of injury.
The authors determined the mechanism for delivering mTOR mRNA to the injured axon. In addition to the part of the mRNA that is translated into protein, mRNA also has a part at the beginning called the 5′-untranslated region (5′-UTR) and a part at the end called the 3′-untranslated region (3′-UTR). Similar to other transcripts that are delivered to axons, delivery of the mTOR mRNA involved information present in the 3′-UTR, which enabled the interaction of the mRNA with proteins that transported the mRNA down the axon (Figure 2). Using genetic engineering with the CRISPR-Cas9 system, Torenzio and colleagues generated mice that lacked the proper 3′-UTR for the mTOR mRNA. These mice had less mTOR mRNA in their sciatic nerves. Their nerves had less injury-induced local protein translation and could not survive injury as well as the nerves of mice with mTOR mRNA with the wild-type (normal) 3′-UTR. Thus, this study showed that axonal delivery of mTOR transcripts and local translation of mTOR is key to the response of peripheral sensory nerves to axonal damage.
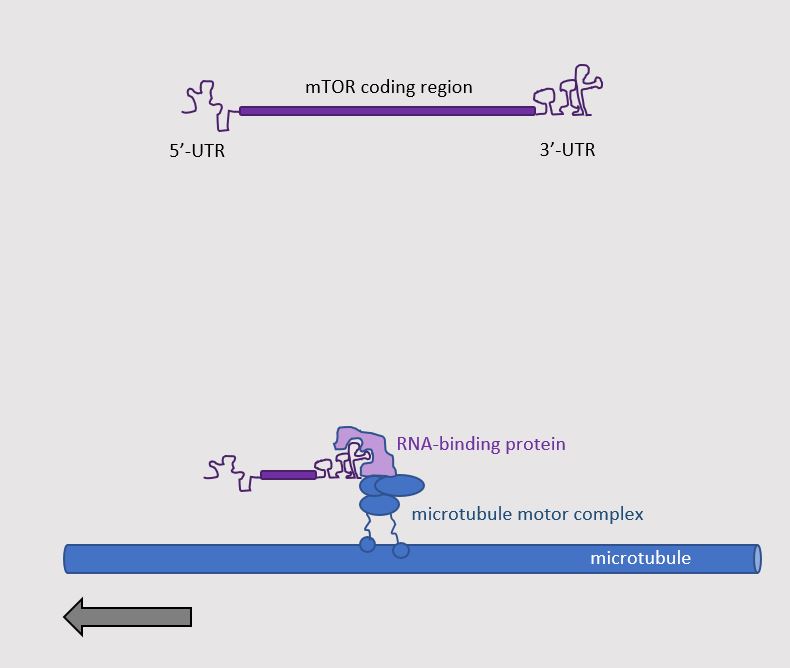
Figure 2. Axonal delivery of mTOR mRNA. [More details]
The second study by Poulopoulos and colleagues represented a technical breakthrough. The Terenzio study examined adult peripheral nerves, which are relatively large compared with the nerves studied by Poulopoulos and colleagues. This second group studied the local proteome (proteins) and transcriptome (mRNAs) in the growth cones of nerves in the developing brains of mice. They used in utero genetic manipulation to express fluorescent proteins and a newly developed sorting technique to separate growth cones from the cell bodies of a specific set of neurons in the developing mouse brain.
The neurons that they studied cross from the cerebral cortex in one hemisphere of the brain to the other, representing a relatively large distance between the extending growth cones and the cell bodies (Figure 3). These neurons are called callosal projection neurons and allow the two hemispheres of the brain to communicate. To study the neurons during development, the scientists injected the brains of the mouse embryos so that they would produce a green fluorescent protein in the nucleus of these callosal projection neurons and a red fluorescent protein that labeled the cell membranes. Three days after the mice were born, the green-labeled cell bodies were isolated from one hemisphere of the brain and the red-labeled membranes were isolated from the other hemisphere. The growth cones in the red-labeled membranes were separated from the rest of neurons on the basis of density and size.
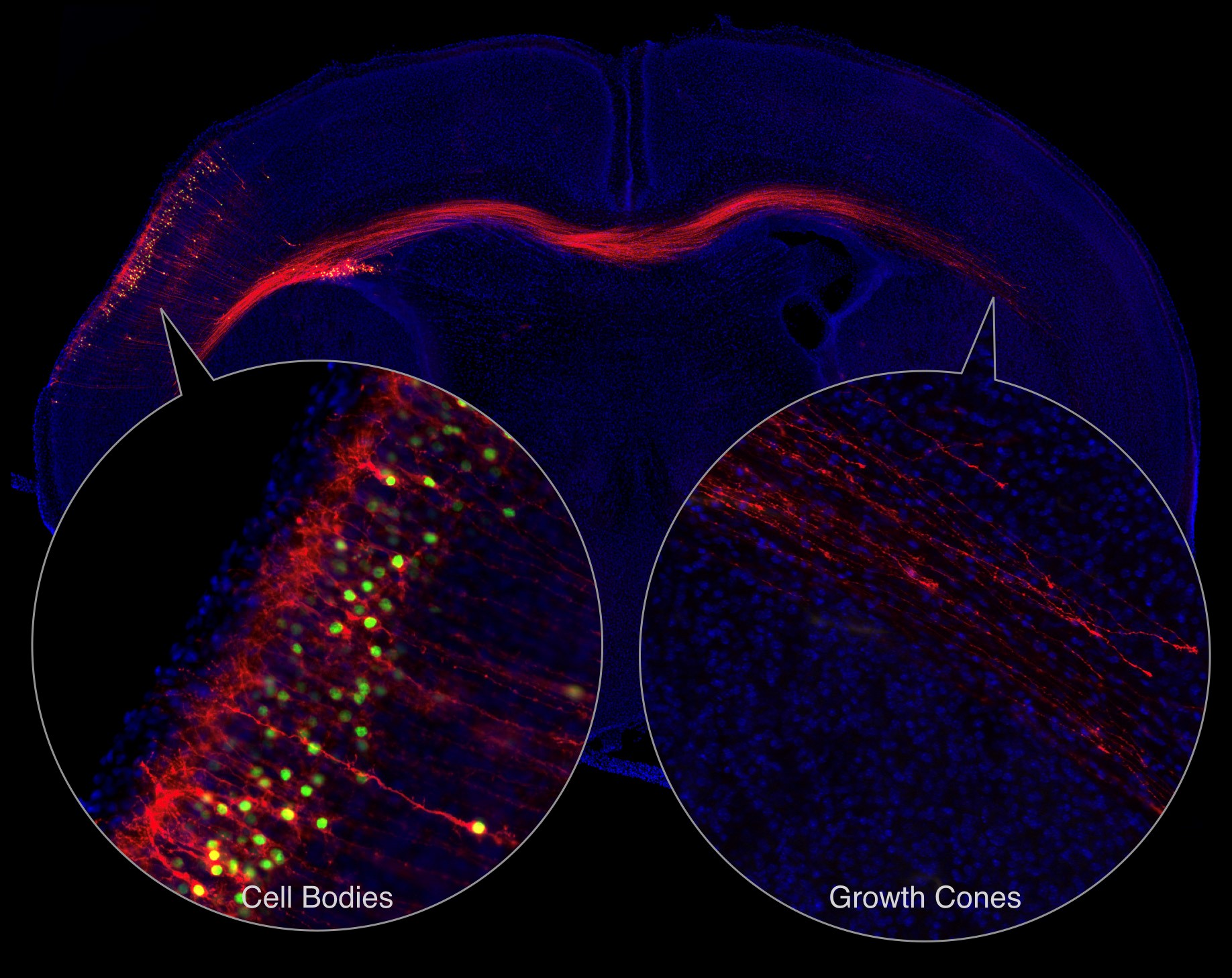
Figure 3. Labeling callosal projection neurons to separate cell bodies from growth cones. [More details]
By analyzing the transcripts present in the cell bodes and those in the growth cones, they found that the growth cones had more transcripts with a 5′-UTR sequence that indicated these transcripts required mTOR for translation. Thus, regulated translation controlled by mTOR appeared especially active in the growth cones of callosal projection neurons. Interfering with mTOR activity disrupted the migration of these neurons across the brain midline in young mice. Thus, not only is mTOR a critical player in the response to adult axon injury, but mTOR is also a key player in growth cones of axons in the developing mammalian brain.
Together the proteomic and transcriptomic data sets from these two studies provide researchers rich resources to investigate not only the developing brain but the response to nerve injury. With the technical advance that enables the analysis of growth cones, detailed understanding the spatial and temporal changes in the proteins of growing axons can be studied. These studies also reveal a similarity between developing neurons in the central nervous system and injured mature neurons of the peripheral nervous system, a critical dependence on mTOR and local translation. This is particularly intriguing, because injured central nervous system neurons do not regenerate effectively; whereas peripheral sensory neurons can. Finding a similar process in these two types of nerves suggests that it may be possible to re-engage developmental processes to restore connections lost to stroke, injury, or neurological disease.
Highlighted Articles
M. Terenzio, S. Koley, N. Samra, I. Rishal, Q. Zhao, P. K. Sahoo, A. Urisman, L. Marvaldi, J. A. Oses-Prieto, C. Forester, C. Gomes, A. L. Kalinski, A. Di Pizio, E. Doron-Mandel, R. Ben-Tov Perry, I. Koppel, J. L. Twiss, A. L. Burlingame, M. Fainzilber, Locally translated mTOR controls axonal local translation in nerve injury. Science 359, 1416-1421 (2018). PubMed
A. Poulopoulos, A. J. Murphy, A. Ozkan, P. Davis, J. Hatch, R. Kirchner, J. D. Macklis, Subcellular transcriptomes and proteomes of developing axon projections in the cerebral cortex. Nature 565, 356-360 (2019). PubMed
Related Reading
J. Nijssen, J. Aguila, R. Hoogstraaten, N. Kee, E. Hedlund, Axon-Seq decodes the motor axon transcriptome and its modulation in response to ALS. Stem Cell Reports 11, 1565-1578 (2018). PubMed
P. K. Sahoo, D. S. Smith, N. Perrone-Bizzozero, J. L. Twiss, Axonal mRNA transport and translation at a glance. J. Cell Science 131, jcs196808 (2018) DOI:
10.1242/jcs.196808 PubMed
T. Shigeoka, H. Jung, B. Turner-Bridger, J. Ohk, J. Q. Lin, P. S. Amieux. C. E. Holt, Dynamic axonal translation in developing and mature visual circuits. Cell 166, 181-192 (2016). PubMed
Related Resources
Poulopoulos Lab, http://poulab.org/ (accessed 17 February 2019)
Molecular Neurobiology Group, Mike Fainzilber, http://www.weizmann.ac.il/Biomolecular_Sciences/Fainzilber/ (accessed 17 February 2019)
Macklis Laboratory, Department of Stem Cell and Regenerative Biology & Center for Brain Science, Harvard University, https://macklislab.hscrb.harvard.edu/ (accessed 17 February 2019)
Cite as: N. R. Gough, The mTOR-Dependent Translatome in Growing Nerves and Injured Axons. BioSerendipity (18 February 2019)
https://www.bioserendipity.com/the-mtor-dependent-translatome-in-growing-nerves-and-injured-axons/.
