Benefits of Off-Target Effects
There are many FDA-approved medicines. Most have some “off-target” effects, meaning effects that are not those intended for the drug. Sometimes these off-target effects limit the dose of a drug that can be used to treat the intended disease or symptom. Sometimes these off-target effects are unwanted side effects that the patient must tolerate, because the benefits of taking the medicine outweigh the harm of the side effects. Sometimes off-target effects can be leveraged to use the drug for a different disease or symptom than the one for which it was originally approved.
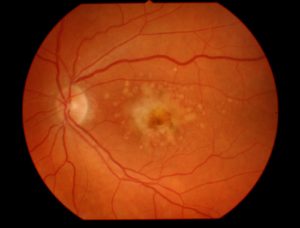
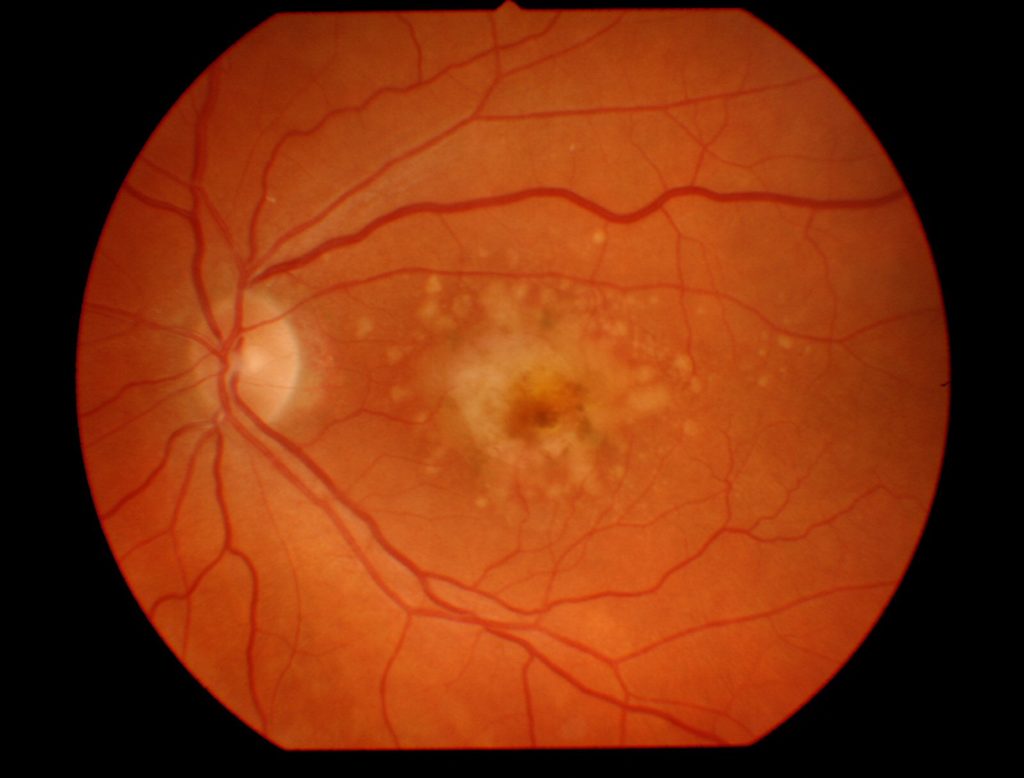
The highlighted paper describes the identification of a new potential therapy to prevent blindness caused by excessive growth of new blood vessels (neovascularization) in the eye. Excess neovascularization in the eye causes blindness associated with premature birth, diabetes, and a type of age-related macular degeneration (AMD) (Figure 1). Basavarajappa and colleagues identified that a drug originally developed as an antifungal agent may be beneficial in preventing blindness (1).
Deep Dive into Ferrochelatase Inhibitors for Preventing Blindness
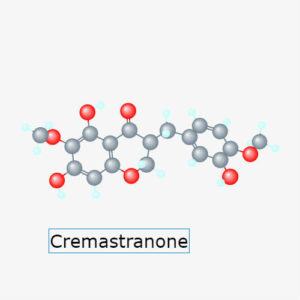
Basavarajappa and colleagues sought the molecular target of a chemical called cremastranone (Figure 2), which inhibits blood vessel growth. Using a process called affinity purification and then mass spectrometry to analyze the bound proteins, the authors determined that cremastranone specifically bound to the protein ferrochelatase from cell lysates. Cremastranone also bound to purified, recombinant ferrochelatase produced by engineered bacteria, indicating that cremastranone bound directly to ferrochelatase without requiring other proteins and not indirectly through another protein or component in the cellular lysates.
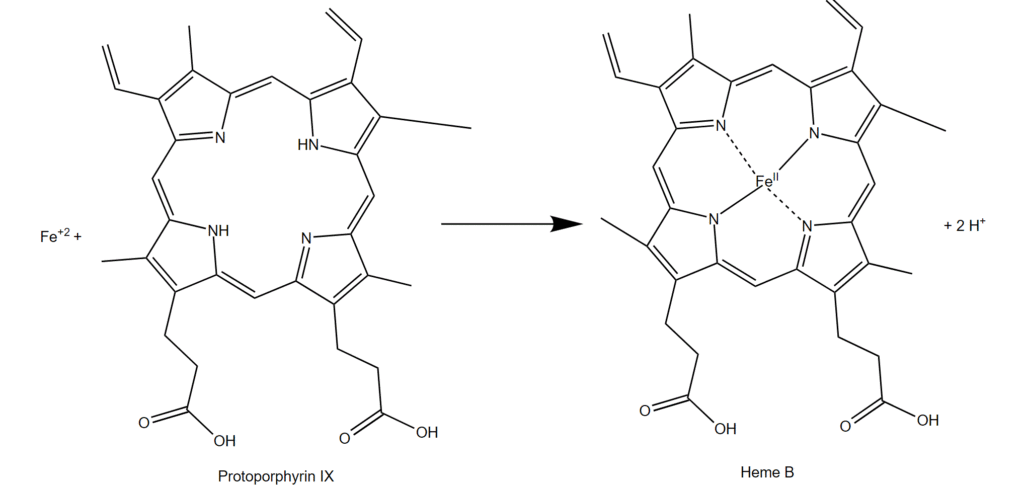
Ferrochelatase is an enzyme that loads iron onto molecules like protoporphyrin to produce heme (Figure 3). All cells have heme-containing proteins. A heme-containing protein in endothelial cells is nitric oxide synthase. When applied to human retinal endothelial cells, which are the cells that form the blood vessels in eye, cremastranone reversibly inhibited proliferation of these cells and caused the accumulation of the substrate of ferrochelatase. Not only did these results suggest that targeting ferrochelatase may be useful in preventing neovascularization-mediated blindness but also indicate that ferrochelatase activity is important for neovascularization.
The authors found that reducing the amount of ferrochelatase in human retinal endothelial cells blocked proliferation, migration, and tube formation of these cells. Applying a known inhibitor of ferrochelatase, N-methyl protoporphyrin (NMPP), had the same effects on these cells. NMPP reduced the amount of nitric oxide synthase and the remaining nitric oxide synthase did not have iron-bound heme. Neither of these methods of reducing ferrochelatase activity caused the cells to die and the effects were reversible. Application of NMPP only impaired proliferation of retinal endothelial cells and human brain microvascular cells, but not umbilical vein endothelial cells or other types of cells from the eye, indicating that not all types of vascular endothelial cells depend on ferrochelatase and that inhibition of this enzyme may have some cellular selectivity. It cannot be completely cellularly selective, because ferrochelatase is important in other cells, such as red blood cells.
The FDA-approved antifungal drug Griseofulvin (Figure 4) forms NMPP and thus also inhibits ferrochelatase activity. This inhibition of ferrochelatase by Griseofulvin is considered an off-target effect. The on-target effect of Griseofulvin is accumulation in skin, hair, and nails through an interaction with keratin (a protein in these tissues) and then disruption of fungal microtubules in the infected area. This disruption of the fungal microtubules impairs fungal growth and replication. Presumably by producing NMPP, Griseofulvin inhibited the proliferation, migration, and tube formation of human retinal endothelial cells. This drug also inhibited sprouting of human microvascular cells of the brain.
Preclinical studies with mice that have laser-induced choroidal neovascularization (L-CNV), which is a model of AMD, showed that feeding the mice Griseofulvin or injecting the drug into the eye reduced new blood vessel growth. Consistent with an important role of ferrochelatase in AMD, post-mortem analysis of eyes from AMD patients and from healthy people of the same age revealed an increase in the amount of ferrochelatase in the eyes from the AMD patients. This study suggests that the antifungal Griseofulvin may be repurposed for AMD and other forms of blindness associated with neovascularization.
Potential Applications of Ferrochelatase Inhibition in Cancer Therapy
The identification of ferrochelatase as a relevant target in the inhibition of angiogenesis suggests that targeting ferrochelatase or other steps in the production of heme may also be relevant in cancer or other conditions associated with aberrant vascularization conditions. Inhibition of ferrochelatase results in the accumulation of protoporphyrin in cells. Protoporphyrin is converted into a toxic product in response to light, thus treatments that result in the accumulation of this molecule cause photosensitivity (light-induced tissue injury) and phototoxicity (light-induced cell death). Indeed, combinations of drugs that induce the accumulation of protoporphyrin, such as deferoxamine (an iron-binding chemical) and 5-aminolevulinic acid (a protoporphyrin precursor) (Figure 5), have been tested as agents that sensitize cells to photodynamic therapy, a treatment that combines light and a photosensitizing drug. Both the photosensitizing and antiangiogenic effects of these drugs may contribute to their potential anticancer effects (2). Thus, ferrochelatase inhibitors may also be useful in this context.
Off-Target Ferrochelatase Inhibition in Kinase Inhibitor Side Effects
Other drugs developed to treat cancer include kinase inhibitors, several of which cause photosensitivity. Klaeger and colleagues tested 226 kinase inhibitors and found that 29 also bound ferrochelatase (3). Further testing of a subset of the 29 showed that the ferrochelatase-binding kinase inhibitors reduced the amount of heme in cells, consistent with inhibition of ferrochelatase activity. These results provide a molecular rationale for the photosensitivity and skin irritation that can be a dose-limiting or treatment-ending side effect of anticancer kinase inhibitors. Understanding this molecular mechanism lets researchers screen kinases against ferrochelatase to identify those that will not trigger the unwanted photosensitivity and skin irritation side effect.
Collectively, these articles demonstrate that one drug’s off-target effect may be leveraged to repurpose that drug for another purpose and also show how understanding the molecular targets of drugs can explain side effects, enabling the developing of drugs lacking the side effects.
Highlighted Articles
- H. D. Basavarajappa, R. S. Sulaiman, X. Qi, T. Shetty, S. P. B. Sheik, K. L. Sishtla, B. Lee, J. Quigley, S. Alkhairy, C. M. Briggs, K. Gupta, B. Tang, M. Shadmand, M. B. Grant, M. E. Boulton. S.-Y. Seo, T. W. Corson, Ferrochelatase is a therapeutic target for ocular neovascularization. EMBO Mol. Med. 9, 786–801 (2017). PubMed
- K. Inoue, H. Fukuhara, A. Kurabayashi, M. Furihata, M. Tsuda, K. Nagakawa, H. Fujita, K. Utsumi, T. Shuin, Photodynamic therapy involves an antiangiogenic mechanism and is enhanced by ferrochelatase inhibitor in urothelial carcinoma. Cancer Sci. 104, 765–772 (2013). PubMed
- S. Klaeger, B. Gohlke, J. Perrin, V. Gupta, S. Heinzlmeir, D. Helm, H. Qiao, G. Bergamini, H. Handa, M. M. Savitski, M. Bantscheff, G. Médard, R. Preissner, B. Kuster, Chemical proteomics reveals ferrochelatase as a common off-target of kinase inhibitors. ACS Chem. Biol. 11, 1245–(2016). PubMed
Cite as: N. R. Gough, Drug Repurposing: Preventing Blindness with an Antifungal. BioSerendipity (10 July 2017). https://www.bioserendipity.com/2017/07/10/drug-repurposing-preventing-blindness-with-an-antifungal/