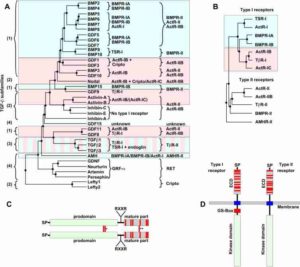
Phylogenetic analysis of the TGF-b superfamily of ligands and receptors. (A) Phylogenetic analysis of the TGF-β ligand superfamily (only the mature region was used) showing the existence of four subfamilies as indicated on the left: BMP/GDF (1), Activin/Inhibin/Nodal (2), TGF-βs (3) and others (4). Type I and type II receptor usage is indicated next to each ligand and deduced from biophysical interaction or in vitro pulldown and crosslinking analyses. Light-blue shaded boxes emphasize SMAD 1/5/8, whereas light-red shaded boxes highlight SMAD 2/3 downstream signaling. (B) Phylogenetic tree (kinase domains were used for analysis) of TGF-β receptors highlighting type I and type II subgroup classification. Color usage for type I receptors indicates the SMAD pathway utilized (as in A). (C) TGF-β ligands are synthesized as dimeric proproteins including an N-terminal signal peptide (SP), a large prodomain and a C-terminal mature part with a characteristic cystine-knot motif. Cysteine residues are illustrated by red bars. Proteolytic processing by furin proteases occurs at the RXXR motif. Indicated by the single asterisk are the two intermolecular disulfide bonds, which are exclusive to TGF-β1/2/3 and covalently link the prodomain dimer. The intermolecular disulfide bond in the dimer of the mature part is marked by two asterisks (Lefty-1, -2, GDF-3, GDF-9 and BMP-15 lack this disulfide bond). (D) The architecture of TGF-β type I and type II receptor architecture consists of an N-terminal extracellular ligand-binding domain (EC), a single span-transmembrane and an intracellular kinase domain. The extracellular parts comprise ten cysteines in both receptor subtypes, but with a distinct sequential arrangement. An intracellular glycine/serine-rich domain (GS-box) characteristic for type I receptors is essential for kinase and downstream SMAD pathway activation. Credit: Mueller and Mickel, FEBS letters 586, 1846-1859 (2012).