Some anti-aging supplements include compounds, like nicotinamide riboside, that increase nicotinamide adenine dinucleotide (NAD: oxidized NAD+ and reduced NADH) and stilbenoid compounds, like resveratrol or pterostilbene, that are anti-oxidants and regulators of protein function. The findings that resveratrol inhibited human platelet aggregation and prevented cancer cell proliferation in culture and cancer in mice created excitement about the benefits of red wine. Resveratrol is present in very small amounts in only a few foods—red wine, grapes, peanuts, and some berries.
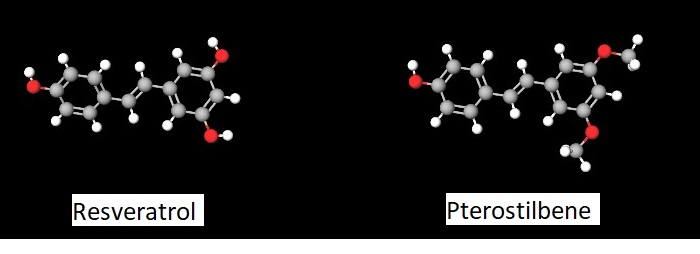
Resveratrol has poor bioavailability when consumed orally. Pterostilbene is chemically related to resveratrol (Figure 1) and found in even smaller amounts to resveratrol in similar foods, such as blueberries, cranberries, lingonberries, and almonds. Compared to resveratrol, pterostilbene is more bioavailable and metabolized less efficiently. These properties of pterostilbene make it attractive for companies developing supplements to promote healthy aging, like Basis by Elysium Health, which contains both nicotinamide riboside and pterostilbene.
Like NAD+, stilbenes can affect multiple cellular processes and may have diverse targets within cells. There is more research into the mechanisms of action and effects of resveratrol than of pterostilbene. However, their functions and targets are likely similar because of their structural and chemical similarity. One target for both compounds is the deacetylase SIRT1 of the sirtuin family. Many of the effects of resveratrol involve activation of the kinase AMPK, which is activated downstream of SIRT1. Differences in cellular metabolic state may dictate whether resveratrol promotes healthy cellular metabolism or triggers cell death (Figure 2).
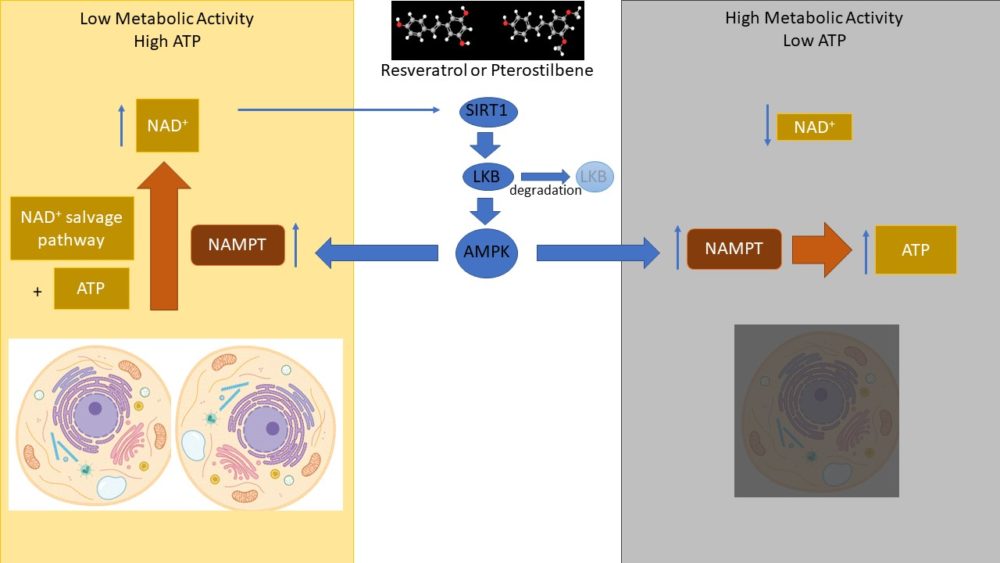
The activation of AMPK stimulates the expression of the gene encoding the enzyme NAMPT. NAMPT can either synthesize NAD+ by mediating a key reaction in the NAD+ salvage pathway, a process that uses the energy of ATP. NAMPT can also mediate the reverse reaction and synthesize ATP. In cells with hyperactive metabolism or metabolism highly dependent on NAD+, such as cancer cells, this can result in an NAD+ crisis and cell death. In cells with low metabolism, such as occurs in diabetes, the increase in NAMPT promotes healthy cellular metabolism through a positive feedback loop involving SIRT1, the LKB1 and AMPK module, the NAD+ salvage pathway, and NAD+, which is required for SIRT1 activity (Figure 2). What may prevent this from becoming a detrimental hyperactive cycle is that LKB1 exhibits regulated degradation.
Stilbenes are also anti-oxidant molecules; they interact with free radicals, such as reactive oxygen species (ROS), thereby preventing these reactive molecules from causing damage by reacting with cellular lipids, proteins, or DNA. Pterostilbene also promotes cellular tolerance to oxidative stress by disrupting an interaction between two proteins: NRF2 and KEAP1. KEAP1 binds to NRF2 and targets NRF2 for degradation. NRF2 is a transcription factor that activates a large set of genes involved in the cellular oxidative stress response. This response also involves expression of genes encoding enzymes that detoxify compounds and thus could contribute to reduced efficacy of medications that are processed by these enzymes, such as anti-cancer drugs. Furthermore, ROS can also be important mediators of cell signals, such as some growth factor signals, or molecules that are important for cellular function, such as the antimicrobial action of macrophages. Thus, it may not be beneficial to chronically eliminate ROS through a stilbene-containing dietary supplement.
These compounds also influence chromatin accessibility by promoting the activity of chromatin-modifying enzymes, such as the sirtuins. Cells exposed to stilbenes have altered DNA methylation patterns and histone acetylation patterns. DNA methylation and histone acetylation represent types of epigenetic modifications that alter gene expression. Consequently, combined with their ability to stimulate the NRF2 transcriptional response, these compounds have complex effects on gene expression that can vary by cell type (such as liver cell, pancreatic cell, muscle cell, and vascular endothelial cell) and cell state (such as stem cell, nondividing cell, actively dividing cell, metabolically active cell, metabolically quiet cell, and cancer cell).
Beneficial effects for resveratrol and pterostilbene have been shown in many animal and cell culture models, and some clinical trials have been done. The vascular, heart, and neurological benefits of these compounds may relate to their antioxidant properties, along with their abilities to regulate cellular pathways involved in the induction of the cellular oxidative stress response, inhibition of platelet aggregation, promotion of blood vessel relaxation through stimulation of nitric oxide (NO) production, and promotion of healthy cellular metabolism by activation of SIRT1 and AMPK. These compounds are also under investigation for use in various metabolic disorders, including diabetes, obesity, and non-alcoholic fatty liver disease. Cancer is another disease for which these compounds are being studied. For some cancers, resveratrol appeared beneficial; for others, there were adverse effects; and for others, there was no effect.
Although well tolerated, it is not yet clear whether chronic use of supplements containing these compounds is universally beneficial or whether there are people who would benefit and people who could be harmed. Because of the ability of these compounds to increase the expression of genes encoding drug-metabolizing enzymes, it is important to be sure that other medications will not be adversely affected by such dietary supplements.
Related Reading
A. A. Bertelli, L. Giovannini, D. Giannessi, M. Bigliori, W. Bernini, M. Fregoni, A. Bertelli, Antiplatelet activity of synthetic and natural resveratrol in red wine. Int. J. Tissue React. 17, 1-3 (1995). PubMed
J. D. Acharay, S. S. Ghaskadbi, Protective effect of pterostilbene against free radical mediated oxidative damage. BMC Complement. Altern. Med. 13, 238 (2013) DOI: 10.1186/1472-6882-13-238. PubMed
B. C. Akinwumi, K.-A. M. Bordun, H. D. Anderson, Biological activities of stilbenoids. Int. J. Mol. Sci. 19, 792- (2018) DOI: 10.3390/ijms19030792. PubMed
A. Y. Berman, R. A. Motechin, M. Y. Wiesenfeld, M. K. Holz, The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 1, 35 (2017) DOI: 10.1038/s41698-017-0038-6. PubMed
E. Bhakkiyalakshmi, K. Dineshkumar, S. Karthik, D. Sireesh, W. Hopper, R. Paulmurugan, K. M. Ramkumar, Pterostilbene-mediated Nrf2 activation: Mechanistic insights on Keap1:Nrf2 interface. Bioorg. Med. Chem. 24, 3378-3386 (2016) DOI: 10.1016/j.bmc.2016.05.011. PubMed
M. Jang, L. Cai, G. O. Udeani, K. V. Slowing, C. F. Thomas, C. W. Beecher, H. H. Fong, N. R. Farnsworth, A. D. Kinghorn, R. G. Mehta, R. C. Moon, J. M. Pezzuto, Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275, 218-220 (1997). PubMed
F. Lan, K. A. Weikel, J. M. Cacicedo, Y. Ido, Resveratrol-induced AMP-activated protein kinase activation is cell-type depndent: Lessons from basic research for clinical application. Nutrients 9, 751, (2017) DOI: 10.3390/nu9070751. PubMed
P. S. Lee, Y. S. Chiou, C. T. Ho, M. H. Pan, Chemoprevention by resveratrol and pterostilbene: Targeting on epigenetic regulation. Biofactors 44, 26-35 (2018) DOI: 10.1002/biof.1401. PubMed
H. Y. Tsai, C. T. Ho, Y. K. Chen, Biological actions and molecular effectos fo resveratrol, pterostilbene, and 3′-hydroxypterostilbene. J. Food Drug. Anal. 25, 134-147 (2017) DOI: 10.1016/j.jfda.2016.07.004. PubMed
J. Tomé-Carneiro, M. Larrosa, A. González-Sarrías, F. A. Tomás-Barberán, M. T. García-Conesa, J. C. Espín, Reseveratrol and clinical trials: The crossroad from in vitro studies to human evidence. Curr. Pharm. Des. 19, 6064-6093 (2013) DOI: 10.2174/13816128113199990407. PubMed
Clinical Trials
Resveratrol, ClinicalTrials.gov (accessed 19 June 2018): https://www.clinicaltrials.gov/ct2/results?cond=&term=resveratrol
Pterostilbene, ClinicalTrials.gov (accessed 19 June 2018): https://www.clinicaltrials.gov/ct2/results?cond=&term=pterostilbene
Compounds
Pterostilbene, PubChem (accessed 19 June 2018) https://pubchem.ncbi.nlm.nih.gov/compound/5281727
Resveratrol, PubChem (accessed 19 June 2018) https://pubchem.ncbi.nlm.nih.gov/compound/445154
Resveratrol, DrugBank (accessed 19 June 2018) https://www.drugbank.ca/drugs/DB02709#targets
Cite as: N. R. Gough, Anti-Aging Supplements III: Berries and Wine for Healthy Aging. BioSerendipity (20 June 2018) https://www.bioserendipity.com/anti-aging-iii/
Also of Interest
Anti-Aging Supplements II: NAD+ as the Key to Healthy Aging
Anti-Aging Supplements: The Basis for Basis