As a pharmacologist, I am skeptical of the benefits touted for many supplements. Most have limited or no clinical trial data showing that they are effective in people who do not have a deficiency in the nutrient or vitamin in the supplement. However, my husband has been taking fish oil capsules for several years. In addition, he is at risk for heart disease. Thus, I was very excited to see the studies showing that a particular fish oil component protects against cardiovascular disease events, such as myocardial infarction, stroke, unstable angina, and the need for stents or cardiac bypass surgery.
The compound tested is icosapent ethyl or ethyl eicosapentaenoic acid. It has an ethyl group attached to the marine fatty acid eicosapentaenoic acid (EPA) and is converted into EPA, an omega-3 fatty acid, in the body (Figure 1).
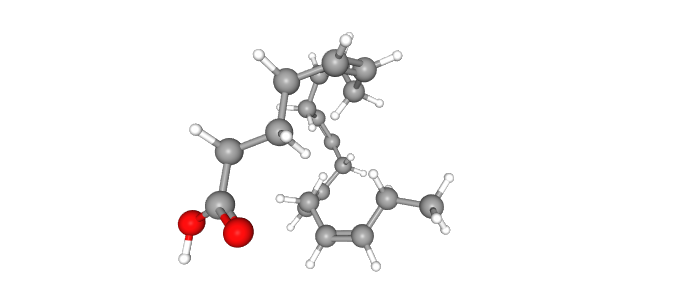 Figure 1. The structure of eicosapentaenoic acid. Read more
Figure 1. The structure of eicosapentaenoic acid. Read more
In this case, the supplement is not just “fish oil,” but a prescription medication (brand name Vascepa) with the active ingredient icosapent ethyl. Currently, Vascepa is FDA-approved for severe hypertriglyceridemia. Trial participants in the test group took 2 g twice per day. The results from the REDUCE-IT clinical trial support the beneficial effects in reducing the risk of a cardiovascular event in patients also receiving moderate or high dose statin treatment.
The clinical trial tested benefits in both preventing primary cardiovascular events, meaning the first episode of an event such as a heart attack or stroke, and secondary events in patients who had already experienced a cardiovascular event. More than 8000 patients participated in the trial, which involved 473 locations and 11 countries. The patients were all receiving statin treatment to lower circulating LDL-cholesterol and either had pre-existing cardiovascular disease or had other risk factors for cardiovascular disease. The comparison was between groups receiving statin + placebo and those receiving statin + icosapent ethyl. More than 5500 patients had existing cardiovascular disease and were in the secondary prevention group; the the remaining were in the primary prevention group. The circulating triglycerides concentration ranged among the participants with some having triglycerides less than 150 mg/dl, some with between 150 and 200 mg/dl and some having greater than 200 mg/dl. Circulating LDL-cholesterol ranged from 41 to 100 mg/dl. The patients were followed for nearly 5 years.
The trial tested for the number of cardiovascular-related deaths and ischemic events: stroke, heart attack, coronary revascularization procedure (stent or bypass surgery), or unstable angina. The icosapent ethyl treatment reduced the number of these events. Additionally, the treatment also reduced the number of subsequent events (second, third, or four or more ischemic events) (Figure 2).
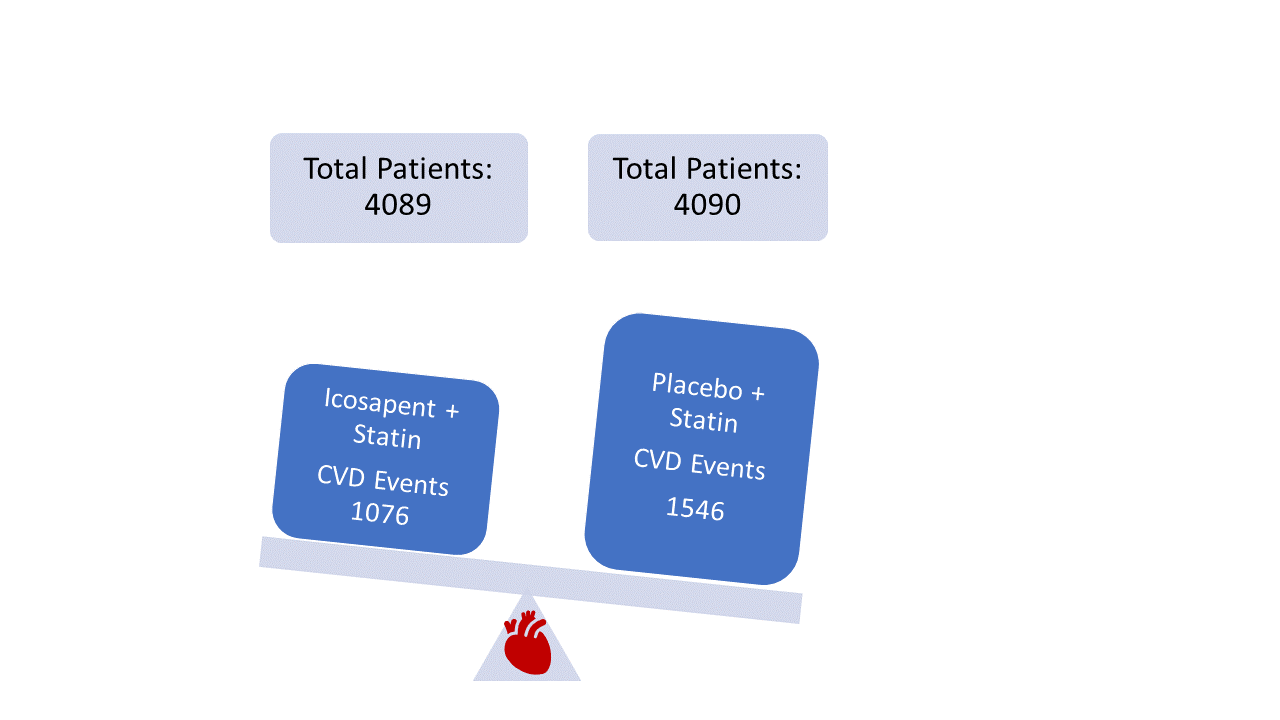
Figure 2. Summary of patient outcomes of REDUCE-IT trial. Read more
Surprisingly, the benefits were observed for all of the patients regardless of their baseline triglyceride levels and regardless of whether the treatment reduced circulating triglycerides below 150 mg/dl. This suggests that the ability to reduce circulating triglycerides may not be the primary mechanism of action. Indeed, this lipid reduces inflammatory markers and platelet activation and stabilizes atherosclerotic plaques.
Another trial, Vitamin D and Omega-3 Trial (VITAL) trial, tested lower doses of omega-3 fatty acid capsules containing the ethyl forms of EPA (460 mg) and docosahexaenoic acid (DHA, 380 mg) did not find a cancer-preventing or cardiovascular event-preventing effect. This trial included >25,000 people, but it did not specifically include or exclude people taking statins. Analysis of the ~8800 participants who were taking statins did not reveal any significant benefit for cardiovascular health. Similar to REDUCE-IT trial, this trial was a randomized controlled trial and followed the participants for ~5 years. However, the dose was less than in the REDUCE-IT trial, and the capsules contained two omega-3 fatty acid ethyl esters, not just the EPA form. These differences could explain the lack of beneficial effect in reducing cardiovascular disease risk between the two trials.
The data from the REDUCE-IT trial support taking a specific dose (2 g twice daily) of icosapent ethyl in patients with a history of cardiovascular disease or risk factors associated with cardiovascular disease and who are already taking statins. What remains to be determined is if the benefits require combination therapy with particular doses of statins for effectiveness. It is worth noting that Amarin, the company that makes Vascepa, funded the REDUCE-IT trial. Based on the results of the REDUCE-IT trial, Amarin has submitted a request to the FDA for expanded use of Vascepa.
Questions that remain for me are:
- How is EPA having this beneficial cardiovascular effect?
- Is combination therapy with a statin required for the beneficial effect?
- Are we missing a key factor in cardiovascular disease by focusing so much on circulating lipids?
Highlighted Articles
D. L. Bhatt, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. New England Journal of Medicine 380, 11-22 (2019). DOI: 10.1056/NEJMoa1812792 PubMed
D. L. Bhatt, et al. Effects of icosapent ethyl on total ischemic events (from REDUCE-IT). Journal of the American College of Cardiology 73, 2791-2802 (2019). DOI: 10.1016/j.jacc.2019.02.032 PubMed
J. E. Manson, et al. Marine n−3 fatty acids and prevention of cardiovascular disease and cancer. New England Journal of Medicine 380, 23-32 (2019). DOI: 10.1056/NEJMoa1811403 PubMed
Clinical Trials
A Study of AMR101 to Evaluate Its Ability to Reduce Cardiovascular Events in High Risk Patients With Hypertriglyceridemia and on Statin. The Primary Objective Is to Evaluate the Effect of 4 g/Day AMR101 for Preventing the Occurrence of a First Major Cardiovascular Event. (REDUCE-IT) https://clinicaltrials.gov/ct2/show/NCT01492361 (accessed 28 October 2019)
Vitamin D and Omega-3 Trials (VITAL) https://clinicaltrials.gov/ct2/show/NCT01169259 (accessed 28 October 2019)
DrugBank
Icosapent, https://www.drugbank.ca/drugs/DB00159 (accessed 28 October 2019)
Icosapent ethyl, https://www.drugbank.ca/drugs/DB08887 (accessed 28 October 2019)
Related Reading
F. Thies, J. M. C. Garry, P. Yaqoob, K. Rerkasem, J. Williams, C. P. Shearman, P. J. Gallagher, P. C. Calder, R. F. Grimble, Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet 361, 477-485 (2003). DOI: 10.1016/S0140-6736(03)12468-3 PubMed
New Indication Approval Sought for Icosapent Ethyl. Pharmacy Times (29 March 2019) https://www.pharmacytimes.com/resource-centers/cardiovascular-health/new-indication-approval-sought-for-icosapent-ethyl
Cover image details
Cite as: N. R. Gough, Statin Plus Icosapent, A Recipe for Reducing Cardiovascular Disease Risk. BioSerendipity (29 October 2019) https://www.bioserendipity.com/statin-plus-icosapent,-a-recipe-for-reducing-cardiovascular-disease-risk/