From Osteoporosis to Breast Cancer
Denosumab, a biologic agent, and bisphosphonates, which are small molecule drugs, reduce fractures that are complications of osteoporosis, including those caused by breast cancer hormone therapies that block estrogen signaling, menopause, or surgically-induced menopause (oophorectomy with or without hysterectomy). Both denosumab and bisphosphonates also reduce the risk of bone metastasis in cancer patients. Denosumab is approved for the treatment of giant cell tumor of the bone and, multiple clinical trials are testing denosumab in various types of advanced cancer that metastasize to the bone.
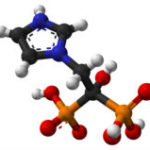
Two bisphosphonates [zoledronic acid (Fig. 1) or clodronate] are recommended for use as adjuvant therapy in postmenopausal (natural or chemically or surgically induced) in breast cancer patients (1), but not yet approved for this use by the FDA. However, only denosumab is appropriate to consider for a breast cancer preventative treatment; because, although both classes of drugs have similar effects in bone, they have different chemical properties, which result in different distributions in the body, and different mechanisms of action.
Both drugs reduce the abundance or function of osteoclasts, the cells that break down bone. Because bisphosphonates bind calcium, these drugs accumulate in the bone, where they are taken up by the osteoclasts. Within the osteoclasts, bisphosphonates disrupt the function of these cells or cause their death.
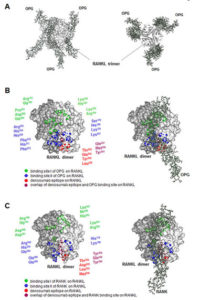
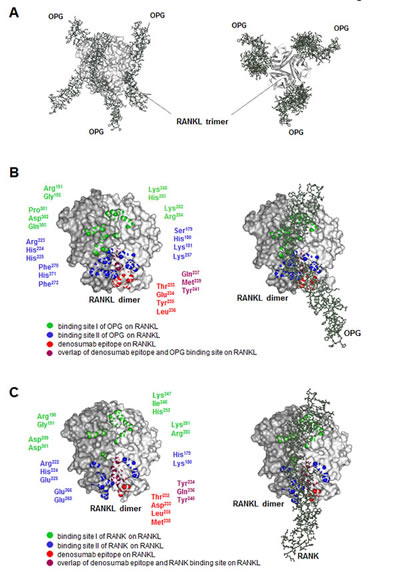
In contrast, denosumab is a monoclonal antibody that binds and inhibits RANKL, which is a natural ligand that stimulates the development of osteoclasts through the receptor RANK (Fig. 2). Therefore, denosumab prevents the development of these cells. Both types of drugs reduce osteolysis (bone degradation) and the potential for bone metastasis, which is often accompanied by osteolysis. However, the RANKL-RANK signaling pathway functions in many other tissues and estrogen and progesterone regulate the activity of this pathway. Thus, denosumab has effects that the bisphosphonates do not.
Because pregnancy requires major changes in a woman’s body, many tissues and organs, not just the breast and uterus, respond to the circulating hormones progesterone and estrogen. Every woman that is having menstrual cycles undergoes changes in her body in preparation for a possible pregnancy. In the breast, these changes include stimulation of breast progenitor cells, their proliferation, and subsequent elimination if pregnancy does not occur (2). This recurrent stimulation and proliferation of breast progenitor cells likely underlies the increase risk of breast cancer with age and the reduction in breast cancer risk with multiple pregnancies. Indeed, the increase in breast cancer incidence in most societies also coincides both with increased longevity and a reduction in the number of pregnancies women have throughout their lifetimes, thereby increasing the number of menstrual cycles and cycles of progenitor cell stimulation and proliferation and, consequently, the chance for a mutation to occur and cancer to develop (3).
In the bone, progesterone stimulates the production of the ligand RANKL, which promotes the development of osteoclasts by binding and activating its receptor RANK. Estrogen stimulates the production of a decoy receptor for RANKL called osteoprotegerin (OPG), thus opposing the action of progesterone in the bone and preventing bone turnover and degradation. The shift in the balance between progesterone and estrogen that occurs during menopause is responsible for increased bone turnover and age-associated osteoporosis in women. Less estrogen enables increased RANKL signaling and increased activity of osteoclasts. Osteolysis is also associated with bone metastasis, thus blocking this process can reduce the risk of bone metastasis. In the bone, denosumab binds RANKL and prevents RANKL from activating its receptor.
In the breast, progesterone stimulates progesterone receptor-positive mammary epithelia cells to produce RANKL. RANKL then stimulates nearby mammary progenitor cells that do not have the progesterone receptor but do have RANK. RANKL stimulates the progenitor cells to divide and RANK-positive basal epithelial cells to make more RANKL. The role of OPG in the breast is less clear, although studies with cells in culture suggest a role in regulating cell death and growth of new blood vessels (4). Every menstrual cycle in women that do not become pregnant is associated with progenitor cell expansion and elimination. Consistent with this mechanism of regulation, risk factors for breast cancer include hormone replacement therapy that includes progesterone, which would promote the proliferation of the progenitor cells, and the number of years a woman has menstrual cycles. High amounts of both circulating RANKL and progesterone correlate with breast cancer risk, and changes in the ratio of RANKL to OPG correlate with the onset of detectable breast cancer and the presence of circulating tumor cells (5).
Blocking RANKL-RANK Signaling to Prevent Breast Cancer
Studies with breast cancer models of mice either lacking RANK in the mammary tissue or given a RANK inhibitor showed that the absence of RANK signaling significantly reduced the occurrence of breast tumors (6). Additionally, analysis of breast tissue from patients with BRCA1 mutations that are associated with breast cancer identified RANK-positive luminal progenitor cells that were highly proliferative, had abnormal DNA repair, and exhibited a molecular signature similar to basal-like breast cancer. Organoids from BRCA1 tissue exhibited reduced progesterone-induced proliferation in response to denosumab, and biopsy tissue showed reduced proliferation in BRCA1 patients given denosumab compared with tissue from patients not given denosumab (6). These results provide a basis for exploring denosumab as a breast cancer prevention strategy, especially in this high-risk group.
Denosumab blocks RANKL-RANK signaling in the breast and in the bone, as well as in other tissues where RANKL-RANK signaling occurs. Denosumab is approved by the FDA as the active biologic in two drugs that differ in their concentration. The lower concentration (Prolia), is approved for use in osteoporosis and to prevent fractures related to cancer therapy. The higher concentration (Xgeva) is approved for preventing bone-related events in patients with bone metastases, patients with giant cell tumor of bone that cannot be surgically removed, and in treating a form of hypercalcemia (too much calcium in the blood). Clinical trials are underway for the use of denosumab as an adjuvant or neoadjuvant treatment in breast cancer, but none of these studies are designed to test if denosumab can prevent breast cancer or delay its appearance. However, a clinical trial to test if denosumab is effective in reducing the development of breast cancer—especially in high risk women, those with BRCA mutations or ductal carcinoma in situ (DCIS)—would be valuable and could reduce the need for prophylactic breast surgeries, including mastectomies. Given that denosumab was approved for use in 2013, there are substantial data indicating the relative safety of this drug. Because the target is RANKL and the mechanism involves limiting the proliferation of a type of stem cell in the breast and osteoclasts in the bone, denosumab could reduce not only breast cancer risk but also reduce fracture risk in women as they age. Even if denosumab only delays the onset of breast cancer until much older age, this effect could reduce the number of deaths from breast cancer, as well as surgeries, radiation treatments, and chemotherapy treatments that so many women receive today.
Related Reading
- S. Dhesy-Thind, G. G. Fletcher, P. S. Blanchette, M. J. Clemons, M. S. Dillmon, E. S. Frank, S. Gandhi, R. Gupta, M. Mates, B. Moy, T. Vandenberg, C. H. Van Poznak, The use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: A Cancer Care Ontario and American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Onc. 10.1200/JCO.2016.70.7257 (6 March 2017). Online Journal
- V. Sigl, L. P. Jones, J. M. Penninger, RANKL/RANK: from bone loss to the prevention of breast cancer. Open Biol. 6: 160230 (2016). PubMed
- C. Tomasetti, L. Li, B. Vogelstein, Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science 355, 1330-1334 (2017). PubMed
- M. Weichhaus, S. T. Chung, L. Connelly, Osteoprotegerin in breast cancer: beyond bone remodeling. Mol. Cancer 14, 117 (2015). PubMed
- S. Kiechl, D. Schramek, M. Widschwendter, E.-O. Fourkala, A. Zaikin, A. Jones, B. Jaeger, B. Rack, W. Janni, C. Scholz, J. Willeit, S. Weger, A. Mayr, A. Teschendorff, A. Rosenthal, L. Fraser, S. Philpott, L. Dubeau, M. Keshtgar, R. Roylance, I. J. Jacobs, U. Menon, G. Schett, J. M. Penninger, Aberrant regulation of RANKL/OPG in women at high risk of developing breast cancer. Oncotarget 8, 3811-3825 (2017). PubMed
- E. Nolan, F. Vaillant, D. Branstetter, B. Pal, G. Giner, L. Whitehead, S. W. Lok, G. B. Mann; Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab), K. Rohrbach, et al., RANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriers. Nat. Med. 22, 933-939 (2016). PubMed
- A. Schieferdecker, M. Voigt, K. Riecken, F. Braig, T. Schinke, S. Loges, C. Bokemeyer, B. Fehse, M. Binder, Denosumab mimics the natural decoy receptor osteoprotegerin by interacting with its major binding site on RANKL. Oncotarget 5, 6647-6653 (2014). PubMed
Clinical Trials
Study of denosumab as adjuvant treatment for women with high risk early breast cancer receiving neoadjuvant or adjuvant therapy (D-CARE). https://clinicaltrials.gov/ct2/show/NCT01077154
Open-label access protocol of denosumab for subjects with advanced cancer. https://clinicaltrials.gov/ct2/show/NCT01419717
Denosumab for breast cancer with bone mets. https://clinicaltrials.gov/ct2/show/NCT01952054
Presurgical trial of denosumab in breast cancer. https://clinicaltrials.gov/ct2/show/NCT02900469
Anakinra or denosumab and everolimus in advanced cancer. https://clinicaltrials.gov/ct2/show/NCT01624766
Cite as: N. R. Gough, Can denosumab prevent breast cancer? BioSerendipity (10 May 2017) https://www.bioserendipity.com/denosumab-breast-cancer/.