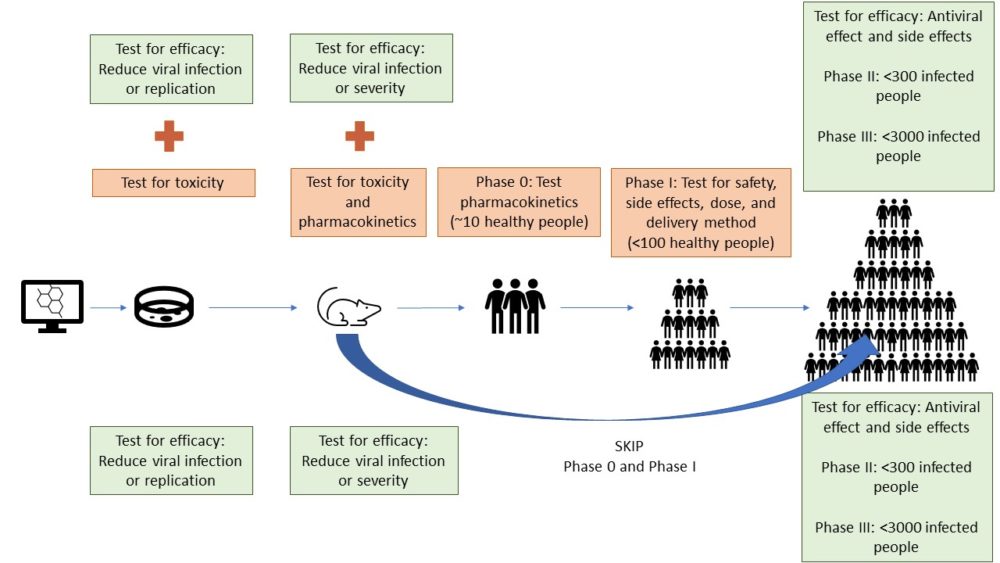
The illustration shows how antiviral agents that have never been used in humans must be tested for both safety and efficacy before being testing in Phase II clinical trials. That is the path outlined in the top part. Candidate antiviral agents that have already been used in humans for other conditions can fast track the process by skipping some of the safety and pharmacokinetic testing. Either they can proceed directly into Phase II or can be tested in combined Phase I/II trials with an adaptive design. This is shown in the bottom of the figure.
Read more: A Fast Track for Testing Antiviral TreatmentsA Fast Track for Testing Antiviral Treatments