Graft-versus-host disease (GVHD) is occurs in people who have received bone marrow or blood stem cell transplants from donors (allogenic transplants). The donated cells replace the stem cells that make the cells of the immune system and red blood cells in patients with leukemias or lymphomas or various types of bone marrow diseases, blood disorders, or metabolic disorders. GVHD is a serious, potentially fatal, reaction that can occur because the immune cells injected with the stem cells or formed from the donor stem cells “see” the recipient as non-self and attack the recipient’s tissues.
GVHD can affect different parts of the body, including the gastrointestinal (GI) tract. The gut contains a very rich diverse set of microbes. The balance of these microbial populations is critically important for maintaining health. Changes in the gut microbiota contribute to the development and severity of GI GVHD. Patients receiving blood cell transplants take medications that compromise the function of the immune system to reduce the chance of GVHD. They may also take antibiotics, antivirals, and antifungals to prevent infection. The preparatory treatment before the transplant and antibiotics given after the transplant alter the microbial populations in the gut, a phenomenon referred to as intestinal dysbiosis.
Patients with GI GVHD have reduced diversity in their gut microbiota. A correlation exists between GI GVHD severity and an increase in the proportion of Enterococci bacteria and a decrease in the proportion of nonpathogenic Clostridia bacteria the gut microbiota. The group nonpathogenic bacteria are referred to as anti-inflammatory Clostridia (AIC). Clinical trials are testing if probiotics, specific diets, and even fecal transplants can treat or prevent intestinal dysbiosis and reduce GVHD severity.
Another idea is to try to shift the balance through diet. Different bacteria can digest different types of sugars. Enterococci are bacteria that digest lactose; whereas the AIC do not. Unpublished work by Stein-Thoeringer and colleagues [presented at the American Society of Hematology (ASH) Annual Meeting in 2018] showed that the post-transplant increase in Enterococci was blocked in mice if the mice were fed a lactose-free diet before and after the transplant. This led the researchers to suggest that a lactose-free diet may be beneficial for blood stem cell transplant recipients. However, dairy products are a source of the beneficial bacteria. So, it is not clear if this dietary change would be effective in people. Additionally, mice were placed on a lactose-free diet before the transplant. In patients, GVHD can occur in two forms: acute GVHD and chronic GVHD. Whether a lactose-free diet would be useful in both types of GVHD is another question. If the research supports limiting lactose to help shift the balance of gut bacteria (reduce Enterococci), then a possibility would be to have patients take a supplement that provides the lactose-digesting enzyme (such as Lactaid, which is used by people who are lactose intolerant). Then, the patients could continue to eat dairy products that contain the beneficial bacteria without providing the sugar source for the harmful bacteria.
Gut bacteria produce metabolites as they digest the foods that we eat (Figure 1). Some bacterial-produced metabolites important for gut health are molecules containing the chemical indole, which are produced from metabolism of the amino acid tryptophan, and short chain fatty acids, which come from the fermentation of digestible fiber.
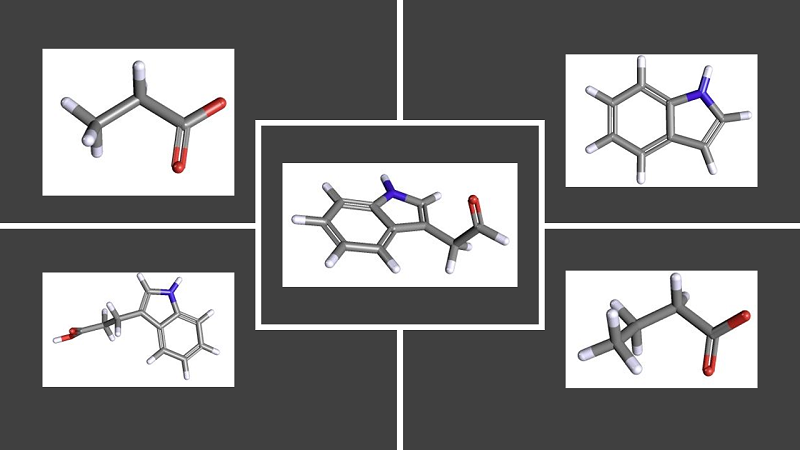
Figure 1. Chemical structures of bioactive metabolites produced by bacteria in the gut [More details]
Two short chain fatty acids that have been implicated in GVHD are butyrate and propionate. Studies in both humans and mice indicate that reduced amounts of these two fatty acids are associated with GVHD and likely result from a decline in the beneficial bacterial AIC populations. In mouse studies, administration of indole or butyrate was protective against GVHD and using the AIC strains as probiotics in these animals also reduced GVHD.
Clearly, there is a connection between the gut microbiota and GI GVHD. Ideally, preserving the microbiota balance in transplant patients would be the best strategy. With the pretreatment regimen and the need for antibiotics, this is challenging. Sufficient studies with humans are lacking to clearly define the best approach. Are there dietary changes that could help patients? Possibly, but those dietary changes probably need to be started before the transplant to be most effective. Once a patient has GI GVHD, the GI tract is damaged and inflamed. After a patient has recovered from GI GVHD, then a diet with limited lactose (to reduce Enterococci) and high in digestible fiber (to increase AIC and short chain fatty acid production) could help maintain a gut microbial population that promotes gut health and limits the chances of a recurrence of GI GVHD.
Related Articles and Reviews
N. T. Baxter, A. W. Schmidt, A. Venkataraman, K. S. Kim, C. Waldron, T. M. Schmidt, Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. mBio 19, e02566-18. DOI: 10.1128/mBio.02566-18 PubMed
N. Köhler, R. Zeiser, Intestinal microbiota influence immune tolerance post allogeneic hematopoietic cell transplantation and intestinal GVHD. Front. Immunol. (2019) DOI: 10.3389/fimmu.2018.03179 PubMed
N. D. Mathewson, R. Jenq, A. V. Mathew. M. Koenigsknecht, A. Hanash. T. Toubai, K. Oravecz-Wilson, S.-R. Wu, Y. Sun, C. Rossi, H. Fujiwara, J. Byun, Y. Shono, C. Lindemans, M. Calafiore, T. C. Schmidt, K. Honda, V. B. Young, S. Pennathur, M. van den Brink, P. Reddy, Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 17, 505-513 (2016). PubMed
L. E. Romick-Rosendale, D. B. Haslam, A. Lane, Lee. Denson, K. Lake, A. Wilkey, M. Watanabe, S. Bauer, B. Litts, N. Luebbering, C. E. Dandoy, S. M. Davies, Antibiotic exposure and reduced short chain fatty acid production after hematopoietic stem cell transplant. Biol. Blood Marrow Transplant. 24, 2418-2424 (2018). PubMed
T. R. Simms-Waldrip, G. Sunkersett, L. A. Coughlin, M. R. Savani, C. Arana, J. Kim. M. Kim. X. Zhan, D. E. Greenberg, Y. Xie, S. M. Davies, A. Y. Koh, Antibiotic-induced depletion of anti-inflammatory Clostridia is associated with the development of graft-versus-host disease in pediatric stem cell transplantation patients. Biol. Blood Marrow Transplant. 23, 820-829 (2017). PubMed
A. Swimm, C. R. Giver, Z. DeFilipp, S. Rangaraju, A. Sharma, A. U. Antonova, R. Sonowal, C. Capaldo, D. Powell, M. Qayed. D. Kalman, E. K. Waller, Indoles derived from intestinal microbiota act via type 1 interferon signaling to limit graft-versus-host disease. Blood 132, 2506-2519 (2018). PubMed
L. S. Zhang, S. S. Davies, Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions. Genome Med. 8, 46 (2016). PubMed
Related Resources
Diseases treatable by transplants. Be the match (accessed 26 February 2019) https://bethematch.org/transplant-basics/how-transplants-work/diseases-treatable-by-transplants/
About graft versus host disease (GvHD). Cancer Research UK (accessed 26 February 2019) https://www.cancerresearchuk.org/about-cancer/coping/physically/gvhd/about
Bone marrow transplant. Mayo Clinic (accessed 26 February 2019) https://www.mayoclinic.org/tests-procedures/bone-marrow-transplant/about/pac-20384854
C. K. Stein-Thoeringer, J. U. Peled. A. L. C. Gomes, A. Lazrak, M. D. Docampo, A. E. Slingerland, K. Nicols, Y. Shono, D. Weber, A. D. Sung, D. Hashimoto, J. Cross, T. Teshima, E. Holler, N. J. Chao, E. G. Pamer, M. R. M. van den Brink, ABSTRACT 358: Intestinal Enterococcus is a major risk factor for development of acute Gvhd. ASH Annual Meeting (2018) https://ash.confex.com/ash/2018/webprogram/Paper119887.html
ASH 2018/Winter 2019 Research Report. Lymphoma Research Foundation (2019) https://www.lymphoma.org/aboutlrf/impact/research/researchreport/
Clinical Trials
Cite as: N. R. Gough, Gut microbes in graft-versus-host disease. BioSerendipity (1 March 2019)
https://www.bioserendipity.com/gut-microbes-in-graft-versus-host-disease/

