The microbes that live in the vagina constitute to the vaginal microbiome (or microbiota). New research from the lab of Jacques Ravel at the University of Maryland shows that Chlamydia infection is associated with a different composition of the vaginal microbiome compared with the microbiome of healthy patients. Additionally, the vaginal microbiome of the patients was changed by antibiotic treatment with azithromycin for the Chlamydia infection. Although the antibiotic cleared the chlamydial infection, the changes to the microbiome were not beneficial. More bacteria that increased the risk of re-infection by Chlamydia were present after the women received azithromycin. One of the bacteria that increased in the population was Lactobacillus iners (L. iners). In contrast, three other species of Lactobacillus (L. crispatus, L. gasseri, and L. jensenii) decreased. Medium from cultures of L. crispatus or L. jensenii were protective against chlamydial infection of cervical epithelial cells in culture. In contrast, medium from L. ines cultures had a very limited effect on infection.
To understand how the different species of Lactobacillus could have such different effects on infection, the researchers looked at the amount of lactic acid produced by the different species. But they didn’t just measure lactic acid, they measured the amount of each chemical form of lactic acid. Like many organic molecules, lactic acid can be produced as an L isomer or a D isomer (Figure 1).
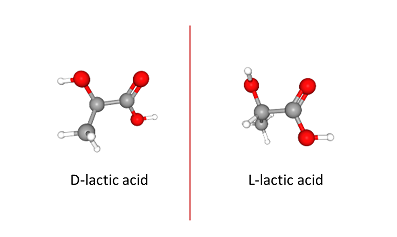
Figure 1. Isomers of lactic acid. View in 3-D and more
Both will reduce the pH in the vagina, which can contribute to preventing pathogenic infections. L. ines produced the L form (L-lactic acid). L. jensenii and L. crispatus produced both forms, but they made more D-lactic acid than L-lactic acid. Exposing the cultured cervical epithelial cells just to D-lactic acid protected them from chlamydial infection, as long as the pH was also low (pH 4). Only reducing the pH without the presence of D-lactic acid was insufficient to prevent infection of the cells. Exposing the bacteria that cause chlamydia infections, Chlamydia trachomatis, to D-lactic acid or L-lactic acid at pH 4 did not impair infection. Thus, the epithelial cells themselves were changed by exposure to low pH and D-lactic acid.
To determine how the epithelial cells were affected by the changes in the vaginal microbiota, the researchers identified small regulatory RNA molecules in samples from 16 women obtained over a 10-week period. In these samples, there were either a preponderance of Lactobacillus species or a diverse array of microbes. The samples with mostly Lactobacillus had high amounts of several small regulatory RNAs called microRNAs. They selected miR-193b to examine in more detail, because this microRNA had a high correlation with vaginal microbiomes dominated by Lactobacillus, and it has well characterized functions in reducing the expression of genes involved in proliferation and cell migration.
The researchers evaluated the amount of this microRNA and the expression of its target genes in cultured vaginal epithelial cells exposed to medium from cultures of L. ines, L. jensenii, or L. crispatus. Consistent with differences in the production of D-lactic acid by the different Lactobacillus, medium from cultures of L. jensenii or L. crispatus, which produced more D-lactic acid than L-lactic acid, reduced CCND1 expression in the cultured vaginal epithelial cells. Medium from cultures of L. iners increased CCND1 expression in the cultured vaginal epithelial cells.
Indeed, vaginal epithelial cells in culture exposed to medium from the different Lactobacillus species showed changes in the expression of many genes, suggesting that microRNAs were not the only regulatory molecules changing. Medium from the D-lactic acid-producing bacteria caused a changes in the expression of genes encoding enzymes that modify chromatin structure to alter gene expression. The genes affected by these enzymes included several involved in regulating cell proliferation. Thus, both changes in microRNA and chromatin structure contributed to the reduction in vaginal epithelial cell proliferation caused by exposure to medium from L. jensenii or L. crispatus cultures. Reduced proliferation, induced with pharmacological inhibitors, protected against infection of cervical epithelial cells. Thus, impaired proliferation is likely a mechanism by which protective Lactobacillus prevent chlamydial infection.
Reducing cell division is not the only possible mechanism by which the species that produce D-lactic acid protect against chlamydial infection. Another mechanism is likely by reducing the expression of the gene encoding the receptor, EGFR, that internalizes Chlamydia trachomatis into cells. Expression of EGFR decreased from 4-22 hours in vaginal epithelial cells exposed to medium from L. jensenii or L. crispatus cultures. Whether D-lactic acid and low pH are sufficient to trigger the changes in chromatin-modifying enzymes or the global changes in gene expression, including the reduction in EGFR expression, was not tested.
However, the data provide potential mechanisms for how changes in the vaginal microbiome, including those associated with antibiotic treatment, can influence susceptibility to sexually transmitted diseases and infections. The changes in the vaginal microbome caused by azithromycin may explain the high rate of recurrence in patients (Figure 2).
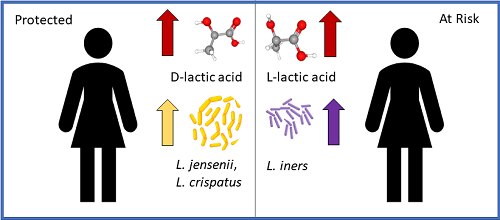
Figure 2. Changes in the microbiome associated with increased risk of recurrent Chlamydia trachomatis infection. Read more
One option to avoid this detrimental change in the microbiome would be to use a different antibiotic. However, studies are needed to determine what antibiotic would be effective against the pathogenic bacteria without disrupting the amount of beneficial bacteria. Another would be to treat with the antibiotic and a vaginal probiotic, similar to how an oral probiotic is often given with an antibiotic to maintain the health of the gut microbiota. As of yet, clinically proven effective vaginal probiotics are not available; but companies, such as LUCA Biologics and OSEL are developing such therapeutics. Indeed, OSEL has a vaginal probiotic product, Lactin-V, that is currently in a clinical trial for recurrent vaginosis.
Highlighted Article
V. L. Edwards, S. B. Smith, E. J. McComb. J. Tamarelle, B. Ma, M. S. Humphrys, P. Gajer, K. Gwilliam, A. M. Schaefer, S. K. Lai, M. Terplan. K. S. Mark, R> M. Brotman, L. J. Forney, P. M. Bavoil, J. Ravel, The cervicovaginal microbiota-host interaction modulates Chlamydia trachomatis infection. mBio 10, e01548–19 (2019). DOI: 10.1128/mBio.01548–19
Related Resources
N. R. Gough, Vaginal Bacteria Guard Against Chlamydia Infection. Medium (19 August 2019).
Lucas Biologics (accessed 17 August 2019).
Osel, Inc (accessed 17 August 2019).
Does your vagina really need a probiotic? Harvard Women’s Health Watch published by Harvard Health Publishing (Harvard Medical School), https://www.health.harvard.edu/womens-health/does-your-vagina-really-need-a-probiotic (accessed 17 August 2019).
Clinical Trial: LACTIN-V Study for Recurrent Bacterial Vaginosis. https://clinicaltrials.gov/ct2/show/NCT02766023 (accessed 17 August 2019).
Cite as: N. R. Gough, Antibiotic Treatment Reshapes the Vaginal Microbiome. BioSerendipity (19 August 2019). https://www.bioserendipity.com/antibiotic-treatment-reshapes-the-vaginal-microbiome/
More articles about host-microbe interactions from BioSerendipity