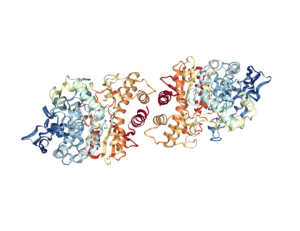
Acetylcholinesterase (AChE) is the enzyme (Figure 1) that degrades the neurotransmitter acetylcholine, which is released at neuronal synapses in the brain, at parasympathetic nerve synapses in the periphery, and at neuromuscular junctions. Acetylcholine activates two classes of receptors, the nicotinic acetylcholine receptor, which is an ion channel, and the muscarinic acetylcholine receptor, which is a G protein-coupled receptor. AChE, which limits the activation of these receptors, is essential for life. Species-specific inhibitors of AChE are used as insecticides. Poisons that inhibit this enzyme in humans have been used as chemical nerve agents. AChE and acetylcholine are also detected at non-neuronal cells, where their functions are just beginning to be understood.
A pair of papers by Spieker and colleagues (1, 2) found a role for AChE in bone development in chickens, ducks, and mice. Pickett and colleagues (3) identified a role for AChE in the development of the intestine in frogs. These studies reveal that there are roles for acetylcholine (cholinergic) signaling, as well as roles for AChE that are independent of acetylcholine, in organogenesis.
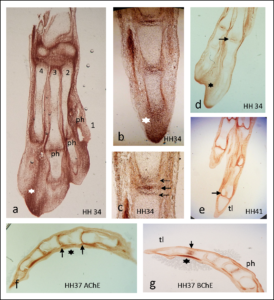
In the first study by Spieker and colleagues (1), antibody staining showed that AChE was highest at the tips of the developing bones and at the location of joint development and was absent from growth plates (Figure 2). The authors used beads soaked in compounds that either stimulated cholinergic signaling or inhibited cholinergic signals. Implanting beads that enhanced cholinergic signaling because the beads had acetylcholine or the enzyme that makes acetylcholine into the developing chicken limb resulted in faster bone growth and mineralization. Unexpectedly, beads soaked in an inhibitor of AChE, which would be predicted to also increase cholinergic signaling, delayed the formation of cartilage and thus impaired bone development. This suggests that AChE has an acetylcholine-independent role in bone development, because the effect of inhibiting its activity was not the same as the effect of adding acetylcholine.
The authors speculate that a second enzymatic activity of AChE may be involved, aryl acylamidase (AAA) activity. AAA activity produces molecules important for the biosynthesis of various biological molecules, including amino acids. The AChE inhibitors that they used (a chemical called BW284c51 and an antibody called MAB304) block this activity of the protein, as well as the acetylcholine-degrading activity and the AAA activity of AChE is high early in chick development.
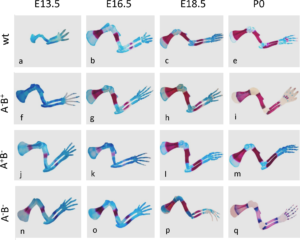
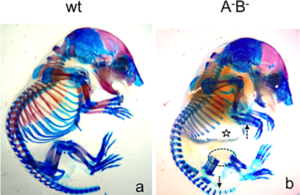
In the second study by Spieker and colleagues (2), the authors used mice in which the genes encoding the enzymes (AChE, BChE, or both) that degrade acetylcholine had been genetically knocked out. AChE is an enzyme that specifically degrades acetylcholine; butyrylcholinesterase (BChE) is a related enzyme that degrades multiple molecules one of which is acetylcholine. This study showed that without these two enzymes cartilage development was abnormal and bone mineralization occurred more quickly than in wild-type mice (Figure 3). Newborn mice lacking these two enzymes had altered extracellular matrix composition of the developing bones, consistent with premature degradation at the site of cartilage at the ends (epiphysis) of the bones. The lack of complete ribs were an obvious defect in skeletal development (Figure 4).
The mutant mice also had altered expression of genes that control development of the skeleton with aberrant expression of individual genes at inappropriate parts of the bone.
Experiments with chicken limb bud cells in culture indicated that AChE, acting by degrading acetylcholine and thus reducing signaling through nicotinic acetylcholine receptors, limited the rate of differentiation and ultimately the death of the cells the form cartilage. Thus, in this context AChE appears to function within the cholinergic signaling system.
In the study by Pickett and colleagues (3), the authors used the frog Xenopus laevis to study the role of AChE in development of the gastrointestinal tract. The authors either exposed the developing frogs to organophosphates that are AChE inhibitors used as pesticides or molecules that reduce the abundance of AChE. During the very earliest stages of development, there are three layers of cells in the embryo: the ectoderm (outermost layer), mesoderm (middle layer), and endoderm (innermost layer). AChE was present in the endoderm of the developing gut.

The intestine of frogs at the early tadpole stage forms a spiral (Figure 5). Blocking AChE throughout the embryo with compounds called morpholino-oligonucleotides or reducing AChE activity only in the endoderm resulted in short guts with abnormal rotation. However, exposing the embryos to carbachol, a drug that activates both muscarinic and nicotinic acetylcholine receptors, or atropine, a drug that inhibits muscarinic acetylcholine receptors, did not affect gut development. Atropine did not rescue gut development in embryos exposed to AChE inhibitors. These results suggest that AChE may function in gut development through a mechanism that does not involve degradation of acetylcholine. Indeed, injecting the RNA for a mutant form of AChE that could not degrade acetylcholine rescued intestinal development in frog embryos injected with the morpholino-oligonucleotides.
Normally, there is only one layer of epithelial cells in the intestinal tract, and these epithelial cells have a polarized morphology with a distinct apical side (top, facing the lumen) and a basolateral side (with the bottom part in contact with the extracellular matrix and the lateral part below the site of cell-cell adhesion). Blocking AChE activity caused the formation of multiple layers of cells and impaired the formation of polarized epithelial cells. To form the polarized epithelial layer, the cells interact with the extracellular matrix and with each other. Using cells in culture, the authors determined that inhibiting AChE did not impair the adhesion of endoderm cells to each other or to the extracellular matrix protein laminin but did impair the adhesion of endoderm cells to the extracellular matrix protein fibronectin. Consistent with a role in mediating fibronectin adhesion, frogs lacking fibronectin have similar gut abnormalities as frogs with reduced AChE.
These studies show that AChE, through both cholinergic-dependent and cholinergic-independent roles, functions during the development of at least two systems—the skeletal system and the gastrointestinal system. Furthermore, although organophosphates may exhibit species-specific selectivity for the catalytic activity of AChE, these poisons may affect the noncatalytic function of AChE in multiple species. This second potential effect of organophosphates may be relevant in both ecology and medicine. Organophosphates used as pesticides could have unintended effects on amphibians, and potentially other animals. In humans, ingestion or exposure to these kinds of pesticides could contribute to birth defects. More studies are necessary to understand if similar cholinergic-independent functions of AChE in organogenesis and interference by organophosphates occurs in other animals, including humans.
Highlighted Research
- Spieker, J., Ackermann, A., Salfelder, A., Vogel-Höpker, A., Layer, P.G., Acetylcholinesterase Regulates Skeletal In Ovo Development of Chicken Limbs by ACh-Dependent and -Independent Mechanisms. PLOS ONE 11, e0161675 (2016). PubMed
- Spieker, J., Mudersbach, T., Vogel-Höpker, A., Layer, P.G., Endochondral Ossification Is Accelerated in Cholinesterase-Deficient Mice and in Avian Mesenchymal Micromass Cultures. PLOS ONE 12, e0170252 (2017). PubMed
- Pickett, M.A., Dush, M.K., Nascone-Yoder, N.M., Acetylcholinesterase Plays a Non-Neuronal, Non-Esterase Role in Organogenesis. Development 144, 2764–2770 (2017). PubMed
Related Resources
Xenbase: The Xenopus model organism database. (accessed on 22 August 2017)
Stage 45 from Xenbase (accessed on 22 August 2017)
RSCB PDB: 3LII (accessed on 22 August 2017)
Cite as: N. R. Gough, Beyond Neurons: Guts and Bones Need Acetylcholinesterase and Cholinergic Signaling to Develop. BioSerendipity (22 August 2017) https://www.bioserendipity.com/2017/08/22/beyond-neurons-guts-and-bones-need-acetylcholinesterase-and-cholinergic-signaling-to-develop/.

