Depression is a complex and common disease. In the US, 1 in 6 people will experience clinical depression sometime in his or her life. There are likely multiple underlying cellular or molecular causes of clinical depression. This is why not all patients respond to any given therapy. Symptoms of clinical depression are diverse and sometimes contradictory. The symptoms include persistent feelings of sadness, hopelessness, or worthlessness; unusual anger or irritability even over small issues; sleep disturbances (either too little or too much sleeping); lack of energy; altered appetite (reduced or increased); anxiety; trouble thinking, concentrating, or remembering; and lack of interest or pleasure in activities that are usually enjoyable. This last symptom of the inability to feel pleasure is called anhedonia.
One of the drawbacks of the approved medicines for treating depression (see list below) is that they take weeks to become effective. Another issue is that many patients do not respond to any of the currently available options for therapy. Weeks of testing one treatment option at a time can lead to months of unsuccessful treatment before all available options are exhausted. The discovery that the anesthetic ketamine had fast-acting antidepressant activity changed the thinking about how depression can be treated and showed that fast-acting therapy is possible.
- Psychotherapy
- Medications
- SSRI, selective serotonin reuptake inhibitors (Prozac, Zoloft, Paxil, Celexa, Lexapro)
- SNRI, serotonin and norepinephrine reuptake inhibitors (Effexor, Pristiq, Cymbalta)
- NDRI, norepinephrine and dopamine reuptake inhibitors (Wellbutrin)
- Serotonin and norepinephrine receptor agonist (Remeron)
- Second-generation antipsychotics (Abilify, Seroquel)
- TCA, tricyclic antidepressants (Elavil, Norpramin, Sinequan, Tofranil, Pamelor, Avantyl, Vivactil)
- MOAi, monoamine oxidase inhibitors (Nardil, Marplan, Parnate, Emsam)
- Experimental or preclinical stage: Ketamine, 4-chlorokynurenine, 2R,6R-HNK, GABA-NAM
- Brain Stimulation Therapy
- Electroconvulsive therapy
- Repetitive transcranial magnetic stimulation
- Vagus nerve stimulation
- Experimental therapy: Deep brain stimulation
Ketamine is used in hospitals as an anesthetic. As such, it is fast acting and not very long lasting. However, in doses lower than those used for anesthesia, a single dose of ketamine can cause symptomatic relief of depression, a reduction in suicidal thoughts, and an improvement in anhedonia within a few hours. Unfortunately, ketamine affects multiple regions in the brain and has several undesirable side effects. Ketamine causes a state called dissociative amnesia, which alters a patient’s sensory perception and ability to remember. This is great for someone having a painful fracture set but not good for patients being treated for depression. Ketamine’s mind-altering ability also makes it a popular recreational drug of abuse, where it is known as “Special K.” Thus, researchers are trying to understand how ketamine works, what specific neuronal circuits are critical for its antidepressant activity, and what other compounds can be used to alter those depression-relevant circuits and only those circuits, rather than the many neuronal circuits affected by ketamine.
Inspired by ketamine, the labs of Todd Gould and Scott Thompson are trying to identify other compounds with fast-acting antidepressant activity. These researchers study rodent models of depression with Gould providing expertise in rodent behavior and Thompson providing expertise in measuring neuronal activity. These two labs and that of their collaborator, Carlos Zarate, Jr., have identified 3 compounds that may be alternatives to ketamine. These compounds are 4-chlorokynurenine, 2R,6R-hydroxynorketamine (2R,6R-HNK), and one called an α5GABA-NAM (Figure 1).
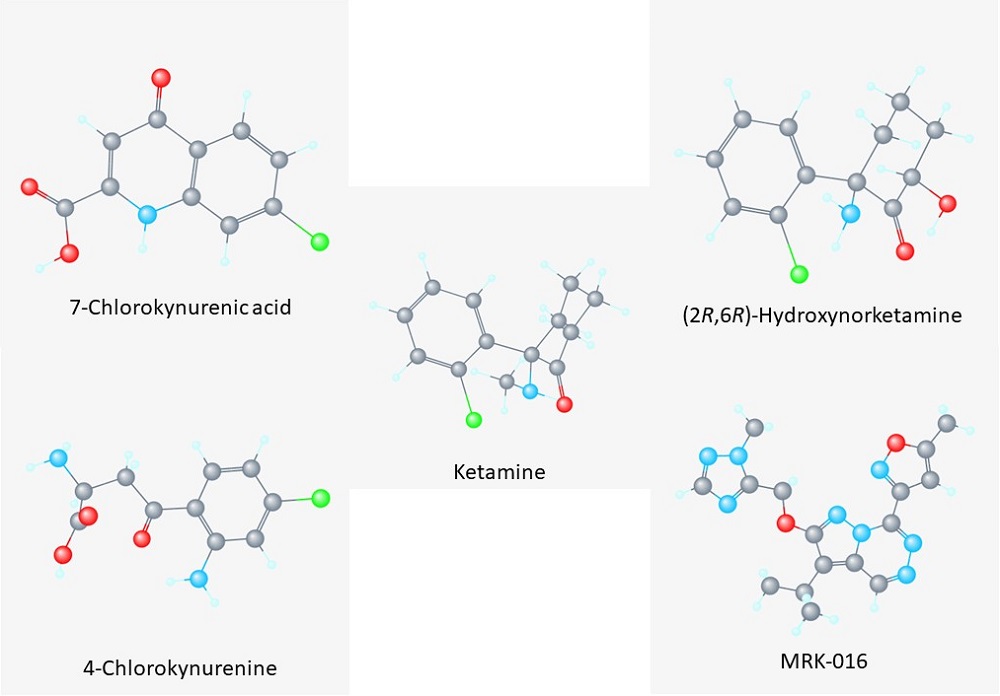
Figure 1. Structures of compounds with fast-acting antidepressant action. [View more details]
Ketamine strengthens excitatory neuronal connections and changes brain activity, which can be monitored by electroencephalography. Ketamine increases gamma waves, which are the highest frequency waves in an EEG. Connections important for the antidepressant effect include those in a circuit involved in the perception of pleasure. The circuit involves neurotransmitter receptors that respond to the natural neurotransmitter glutamate. One type of glutamate receptors are called AMPA receptors, which consist of a specific set of proteins and form a channel in the cell membrane when activated. When the AMPA receptors are abundant at the synapse (the place where the two neurons communicate) and are activated, the neuron receiving the signal is stimulated and the strength of this excitatory synapse is enhanced. Persistent strengthening of specific excitatory synapses in the brain’s reward pathway is part of the way that we use to remember pleasurable experiences and desire to repeat those experiences. Animal models of depression show that these synapses cannot be strengthened properly in response to stimulation; the synapses do not undergo a process called long-term potentiation (LTP) in the animals that have symptoms and behaviors reflecting depression.
One of the proteins involved in the antidepressant responses to ketamine is another type of glutamate receptor called the NMDA receptor. This receptor is activated when both glutamate and glycine are bound and also transmits excitatory signals to neurons. Ketamine may bind to NMDA receptors and inhibit their activity. The chemical 4-chlorokynurenine crosses the blood-brain barrier and is converted into the active molecule 7-chlorokynurenic acid (7-Cl-KYNA). 7-Cl-KYNA interferes with the ability of glycine to bind to the NMDA receptor. Thus, like ketamine, this chemical inhibits the NMDA receptor. Clinical trials are in process testing 4-chlorokynurenine as an antidepressant.
Ketamine is also metabolized in the body to active compounds. One of these is 2,6-hydroxynorketamine (2,6-HNK), which like ketamine, exists in two chemical forms an R form and an S form. The R form, 2R,6R-HNK, has the antidepressant properties without the dissociative and sensory-altering effects of ketamine, which contains both the S and R forms. In contrast to ketamine, 2R,6R-HNK does not bind to the NMDA receptor. Like ketamine, the antidepressant activity of 2R,6R-HNK requires the activity of the AMPA receptor and enhances the activity of this receptor and strengthens this synapse in the reward pathway. Like ketamine, 2R,6R-HNK increases gamma waves in EEG recordings (Figure 2). Gould is working with his collaborators at the NIH to begin clinical trials testing the safety of this compound and, if safe, to test its effectiveness in treating depression.
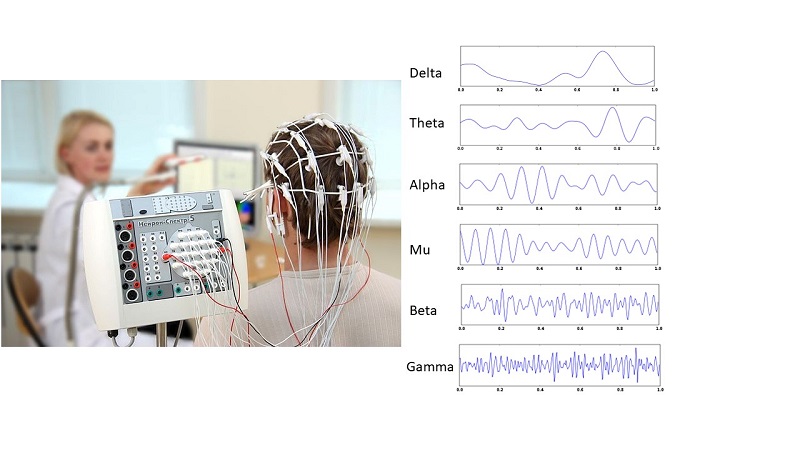
Figure 2. EEG recordings. Ketamine and compounds with fast-acting antidepressant activity increase the frequency of gamma waves. [View more details]
The reward circuit involves more than just the excitatory synapse, NMDA receptors, and AMPA receptors. There are also inhibitory neurons that control the activity of the circuit. If those inhibitory neurons are too active, this can also prevent synaptic strengthening of the excitatory connections. Indeed, one of the proposed mechanisms for how ketamine exerts its antidepressant effects is by reducing the activity of the inhibitory neurons in this pathway. Thompson identified a set of receptors that are specifically present on these inhibitory neurons in the reward pathway. These receptors bind the neurotransmitter GABA, which reduces neuronal activity when it binds and stimulates GABA receptors and activates the inhibitory neurons. There are many subtypes of GABA receptors; the ones with the α5 subunit are present in the inhibitory neurons of the reward pathway. Thus, Thompson sought chemicals that would specifically interact with GABA receptors with the α5 subunit, which led to the identification of the α5GABA-NAM called MRK-016. This GABA-NAM effectively alleviated stress-induced depression behaviors in mice within 24 hours and strengthened the same neuronal connection that is strengthened by a single administration of ketamine or 2R,6R-HNK. Importantly, this α5GABA-NAM produced none of the adverse effects of ketamine. He has launched a biotechnology company, Asulon Therapeutics, to develop α5GABA-NAM compounds with suitable properties for clinical development that can ultimately be tested in clinical trials for depression.
Whereas Gould’s compound 2R,6R-HNK targets the excitatory synapse in the reward pathway directly; Thompson’s α5GABA-NAM strengthens this connection indirectly by reducing an inhibitory input (Figure 3). Best of all, these compounds achieve this strengthening of the circuit after a single treatment in model animals without the adverse effects observed with ketamine. Although both researchers study the same disease, they are not competitors. As Thompson states, “Whether Dr. Gould’s drug turns out to be the silver bullet or whether mine does or whether both are doesn’t matter. What matters most is that our research leads to improved treatment options for patients with depression.”
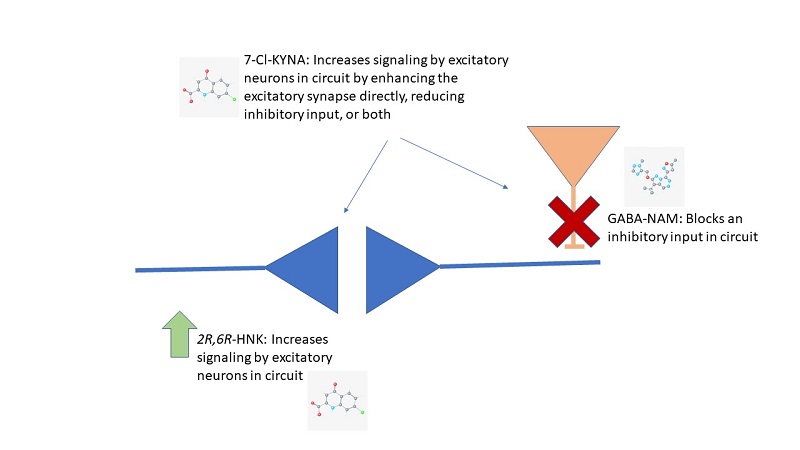
Figure 3. Direct and indirect actions of fast-acting antidepressants at synapses in the reward pathway. The blue neurons represent an excitatory synapse and the orange neuron provides inhibitory input. [View more details]
Through the efforts of researchers such as Thompson and Gould, there is hope that fast-acting antidepressants will be available soon. Such drugs will be helpful as first line treatment for patients who have attempted suicide or have been hospitalized for thoughts of suicide. Such options will give clinicians and mental health care providers valuable tools for rapidly helping severely depressed patients and those who do not respond to other treatments.
Related Resources
Depression (major depressive disorder). Mayo Clinic https://www.mayoclinic.org/diseases-conditions/depression/symptoms-causes/syc-20356007 (accessed 4 January 2019).
Asulon Therapeutics, Inc. Wins “Best in Show” at Baltimore 1st Pitch Life Science Competition http://www.1stpitchlifescience.com/asulon-therapeutics-inc-wins-best-in-show-at-baltimore-1st-pitch-life-science-competition/ (accessed 18 February 2020)
Labs
Gould Lab https://gouldlab.weebly.com/ (accessed 28 December 2018).
Scott M. Thompson, University of Maryland School of Medicine, Faculty Profile http://www.medschool.umaryland.edu/profiles/Thompson-Scott/ (accessed 28 December 2018).
Carlos A. Zarate, Jr., National Institute of Mental Health, Experimental Therapeutics & Pathophysiology Branch https://www.nimh.nih.gov/labs-at-nimh/principal-investigators/carlos-zarate.shtml (accessed 9 January 2019)
Investigational Drugs
Ketamine
National Center for Biotechnology Information. PubChem Compound Database; CID=3821, https://pubchem.ncbi.nlm.nih.gov/compound/3821 (accessed 9 January 2019).
α5GABA-NAM (MRK-016)
National Center for Biotechnology Information. PubChem Compound Database; CID=6918583, https://pubchem.ncbi.nlm.nih.gov/compound/6918583 (accessed 9 January 2019 ).
(2R,6R)-Hydroxynorketamine hydrochloride
National Center for Biotechnology Information. PubChem Compound Database; CID=121513910, https://pubchem.ncbi.nlm.nih.gov/compound/121513910 (accessed 9 January 2019 ).
4-Chlorokynurenine
National Center for Biotechnology Information. PubChem Compound Database; CID=9859632, https://pubchem.ncbi.nlm.nih.gov/compound/9859632 (accessed 9 January 2019).
7-Chlorokynurenic acid
National Center for Biotechnology Information. PubChem Compound Database; CID=1884, https://pubchem.ncbi.nlm.nih.gov/compound/1884 (accessed Jan. 11, 2019).
Clinical Trials
Antidepressant Effects of the Glycine Receptor Antagonist AV-101 (4-chlorokynurenine) in Major Depressive Disorder. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02484456 (accessed 9 January 2019)
Research Articles
T. D. Gould, C. A. Zarate, Jr., S. M. Thompson, Molecular pharmacology and neurobiology of rapid-acting antidepressants.
Annu. Rev. Pharmacol. Toxicol. 59, 213-236 (2019). PubMed
J. N. Highland, P. J. Morris, P. Zanos, J. Lovett, S. Ghosh, A. Q. Wang, C. A. Zarate, Jr., C. J. Thomas, R. Moaddel, T. D. Gould, Mouse, rat, and dog bioavailability and mouse oral antidepressant efficacy of (2R, 6R)-hydroxynorketamine. J. Psychopharmacol. (2018) DOI: 10.1177/0269881118812095 PubMed
A. C. Nugent, E. D. Ballard, T. D. Gould, L. T. Park, R. Moaddel, N. E. Brutsche, C. A. Zarate, Jr., Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol. Psychiatry (2018) DOI: 10.1038/s41380-018-0028-2 PubMed
P. Zanos, M. E. Nelson, J. N. Highland, S. R. Krimmel, P. Georgiou, T. D. Gould, S. M. Thompson, A negative allosteric modular for
α5 subunit-containing GABA receptors exerts a rapdi and persistent antidepressant-like action without the side effects of the NMDA receptor antagonist ketamine in mice. eNeuro. 4, ENEURO.0285-16.2017 (2017). PubMed
P. Zanos, R. Moaddel, P. J. Morris, P. Georgiou, J. Fischell, G. I. Elmer, M. Alkondon, P. Yuan, H. J. Pribut, N. S. Singh, K. S. Dossou, Y. Fang, X. P. Huang, C. L. Mayo, I. W. Wainer, E. X. Albuquerque, S. M. Thompson, C. J. Thomas, C. A. Zarate, Jr., T. D. Gould, NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533, 481-486 (2016). PubMed
P. Zanos, S. C. Piantadosi, H. Q. Wu, H. J. Pribut, M. J. Dell, A. Can, H. R. Snodgrass, C. A. Zarate, Jr., R. Schwarcz, T. D. Gould, The prodrug 4-chlorokynurenine causes ketamine-like antidepressant effects, but not side effects, by NMDA/GlycineB-site inhibition. J. Pharmacol. Exp. Ther. 35, 76-85 (2015). PubMed
Also of Interest in BioSerendipity
Giving Mice Artificial Memories to Discover Clues to Depression
Cite as: N. R. Gough, Discovering fast-acting treatments for depression. BioSerendipity (11 January 2019) https://www.bioserendipity.com/discovering-fast-acting-treatments-for-depression/.