Some diseases arise because the immune system reacts with normal, healthy cells in the body. In other diseases, lack of an effective immune response contributes to the disease. Cancer cells escape attack by the immune system, in part, by producing a lot of the protein PD-L1 (Gough, 24 May 2017). PD-L1 binds and activates the immunosuppressive receptor PD-1 on T cells that would normally attack the cancer cells, and PD-L1 also interacts with PD-1 on macrophages to prevent these immune cells “eating” the cancer cells. Cancer therapies that disrupt this interaction are currently either clinical trials or approved for treating some forms of cancer (Gough, 22 May 2017). A potential hazard associated with these therapies is excessive immune reactivity toward healthy cells. Indeed, inappropriate autoreactive immune cells cause type 1 diabetes (T1D).
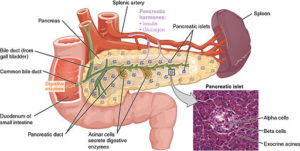
Ban Nasr and colleagues (1) used a mouse model of obesity-independent T1D (hyperglycemic NOD mice). Hyperglycemic NOD mice had much less transcript and protein for PD-L1 on the hematopoietic stem cells isolated from the bone marrow than did stem cells from the same type of mice that were not diabetic (normoglycemic NOD mice) or other types of mice that did not develop diabetes. Hematopoietic stem cells are the cells that make the different kinds of blood cells. The authors induced the production of PD-L1 in the stem cells in culture in two ways—either by genetic engineering using a virus with PD-L1 or by a pharmacological approach—and then injected the cells into the diabetic NOD mice. Either the gene therapy approach with the virus or the pharmacological approach resulted in cells that exhibited immunosuppressive properties when tested in culture. Importantly, when injected into the diabetic mice, the stem cells with induced PD-L1 expression reversed the hyperglycemia, reduced infiltration of the pancreas by immune cells, and reduced inflammation of the part of the pancreas containing the β cells.
This cell-based treatment did not impair all immune responses and exhibited specificity for the diabetic condition. The injected mice mounted an immune response when injected with an irritant. The injected induced cells migrated into the pancreas in the hyperglycemic NOD mice but not in normoglycemic NOD mice. The injected cells appeared to differentiate into myeloid cells (a kind of white blood cell) that could suppress autoreactive T cells and mice receiving the cells had an increase in regulatory T cells that also suppress T cells. Another potentially important finding from the mouse data is that PD-L1 abundance decreased with age, which may explain the occurrence of LADA in human patients.
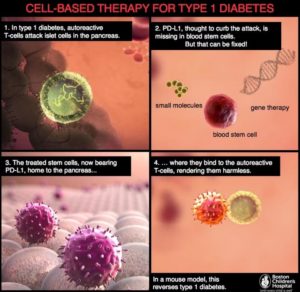
To show that these findings in mice were relevant to human T1D, the authors compared hematopoietic stem cells from patients with T1D and healthy controls. The abundance of PD-L1 was reduced at both the transcript and protein levels in these cells from the diabetic patients. Furthermore, the same pharmacological cocktail [interferon γ, interferon β, and poly(I:C)] that was used in the mouse study increased the amount of PD-L1 present in the cultured patient-derived cells and stimulated their immunosuppressive properties toward cells that reacted with a protein from pancreatic islet cells. These data indicated that pretreating hematopoietic stem cells with this cocktail or using a gene therapy approach and transplanting them back into the patients with T1D may increase the effectiveness of this cell-based therapy (Figure 2).
This study represents a different twist on engineering the immune system compared with the engineered cell-based therapies used for treating cancer, such as CAR T-cell therapy. In contrast to CAR T-cell therapy with the goal of attacking cells in the body (cancer cells), this cell-based therapy has the goal of preventing the immune system from attacking cells of the body (pancreatic β cells). Furthermore, whereas cancer immunotherapy targeting PD-L1 inhibits this immune checkpoint pathway; here, the immunotherapy for diabetes strives to enhance the function of the PD-L1-dependent immune checkpoint.
Highlighted Article
M. B. Nasr, S. Tezza, F. D’Addio, C. Mameli, V. Usuelli, A. Maestroni, D. Corradi, S. Belletti, L. Albarello, G. Becchi, G. P. Fadini, C. Schuetz, J. Markmann, C. Wasserfall, L. Zon, G. V. Zuccotti, P. Fiorina, PD-L1 genetic overexpression or pharmacological restoration in hematopoietic stem and progenitor cells reverses autoimmune diabetes. Sci. Transl. Med. 9, eaam7543 (2017). PubMed
News and Commentary
N. Fliesler, A step toward diabetes immunotherapy. Harvard Medical School (15 November 2017) https://hms.harvard.edu/news/step-toward-diabetes-immunotherapy
N. R. Gough, Overriding the immune system’s brakes without crashing. BioSerendipity (22 May 2017). https://www.bioserendipity.com/2017/05/22/understanding-the-complexity-of-cancer-immunotherapy/
N. R. Gough, Understanding immune checkpoint pathways to improve patient response. BioSerendipity (24 May 2017). https://www.bioserendipity.com/2017/05/24/understanding-im…patient-response/
N. R. Gough, Fighting cancer with the immune system: Lessons from CAR T cell therapy. BioSerendipity (15 May 2017). https://www.bioserendipity.com/2017/05/15/lessons-from-car-t-cell-therapy/
Genes and Proteins
PDL1, UniProt (accessed 5 December 2017) http://www.uniprot.org/uniprot/Q9NZQ7
Cite as: N. R. Gough, Engineering the immune system to treat diabetes. BioSerendipity (11 December 2017). https://www.bioserendipity.com/engineering-the-immune-system-to-treat-diabetes/