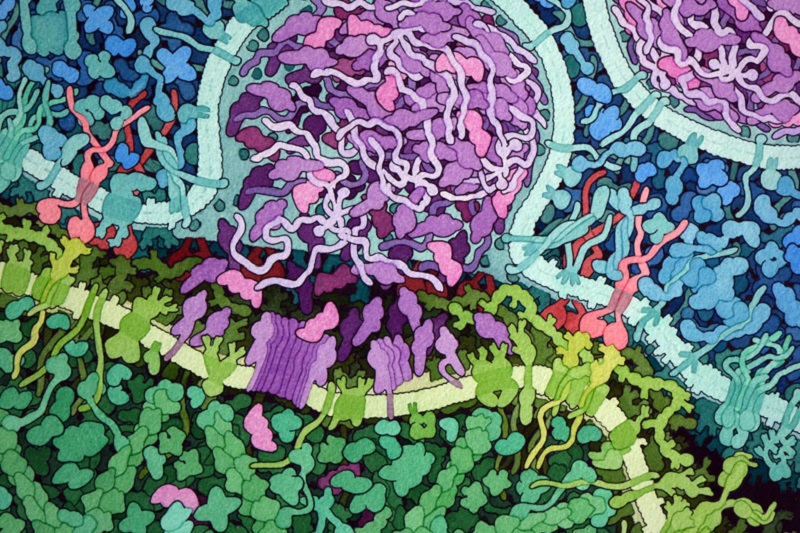
The Promise of CAR T Cell Therapy
Many strategies that train the immune system to recognize and attack cancer are in development or undergoing clinical trials (1). However, these strategies have the potential to cause life-threatening consequences, as evidenced by the latest report of the death of another patient receiving CAR T cell therapy (2). CAR T cell therapy involves using synthetic biology to engineer a chimeric antigen receptor (CAR) that recognizes an epitope (an antigenic part of a protein) on the cancer cells. This CAR is then expressed in the patient’s own T cells and those cells are grown in culture and reintroduced back into the patient (Figure 1). The early successes with this approach led Science Signaling to hail it as a breakthrough of the year in 2012 (3). In fact, several companies, such as Cellectis (France), Kyte Pharma (with headquarters in both the US and the Netherlands), and Novartis (US and Switzerland), are developing CAR T cell therapeutics. Presently, CAR T cell therapies are only effective in blood cancers, such as acute lymphoblastic leukemia and chronic lymphocytic leukemias, but researchers are actively pursuing strategies to apply this approach in patients with solid cancers.
The Reality of Toxicity of CAR T Cell Therapy
Toxicity to CAR T cell therapy can come from a patient that responds too effectively—called cytokine release syndrome. An effective treatment for cytokine release syndrome is giving the patient the biologic agent tocilizumab, which blocks the interleukin-6 (IL-6) receptor, thereby reducing the detrimental consequences of the release of the cytokine IL-6 that is triggered by the CAR T cells attacking the cancer cells (4). Potentially, cytokine release syndrome will be less problematic when CAR T cell therapy is applied to solid cancers. This is because either (i) the right combination of CAR T cell and immune-suppressing therapy can be used or (ii) solid cancers will respond more slowly compared with the rapid, massive response that can occur when blood cancers are treated with CAR T cells.
The Resistance to CAR T Cell Therapy
For solid cancers, signals produced by the tumor microenvironment can also produce resistance. Once again, synthetic biology may provide a solution. Mohammed et al. found that CAR T cells exposed to interleukin-4 (IL-4) did not proliferate as effectively as the same cells exposed to interleukin-2 (IL-2) (5). With knowledge of cytokine signaling systems, the authors engineered a CAR that recognized an epitope on the cancer cells and a chimeric cytokine receptor. These dually engineered CAR T cells now proliferated effectively in response to the IL-4 produced by the cancer cells. Furthermore, the CAR T cells required both activation of the CAR and of the chimeric cytokine receptor to survive and proliferate, which could serve as a useful check on the system reducing toxicity.
To avoid resistance resulting from T cell exhaustion, as Crystal L. Mackall (Stanford University) discussed in her presentation at AACR 2017 (6), scientists need a better understanding of the regulatory pathways that control this immune process. Once again, synthetic biology may hold a solution. The CAR T cells can be engineered to only form functional signaling complexes to trigger tumor cell toxicity in the presence of a second drug (7) or can be engineered so that the CAR is unstable unless a second drug is present (6). Either of these strategies would enable the doctor to “turn on” the CAR T cells by giving the second drug to the patient, then turn the CAR T cells off again by discontinuing the drug, thus preventing exhaustion and potentially reducing toxicity.
The Need for More Science
The problem of T cell exhaustion may be particularly important for strategies aimed at re-educating the immune system to recognize solid cancers. These cancers are often associated with inflammation. Consequently, the adaptive immune cells that infiltrate the tumor (tumor-infiltrating lymphocytes) may have become exhausted or functionally impaired. As with all regulatory mechanisms in the immune system, T cell exhaustion keeps the immune system in balance. Blocking or impairing T cell exhaustion could trigger an autoimmune condition or an excessive response to infection or injury. Thus, treatments aimed at reactivating these cells or preventing T cell exhaustion can potentially tip the delicate immune balance. This is especially true if the treatments reactivate any exhausted T cell, not just the tumor-recognizing T cells. The key to devising tumor-specific therapies is to understand the immune system of the tumor environment and how the tumor alters the immune response. A pair of studies leverages mass cytometry methods to catalog the immune landscape of renal cell carcinoma or lung adenocarcinoma (9, 10). Although studies like this pair are valuable, we also need those that provide the details of the microenvironment and immune profile of other solid cancers. Studies of the environment and immune cells associated with metastatic sites or disseminated tumor cells are also needed. Acquiring and compiling all of this information will point to new therapeutic strategies.
Related Reading
- R.-F. Wang, H. Y. Wang, Immune targets and neoantigens for cancer immunotherapy and precision medicine. Cell Research 27, 11-37 (2017). PubMed https://www.ncbi.nlm.nih.gov/pubmed/28025978
- R. Robbins, Patient dies from severe brain swelling after taking Kite’s CAR-T therapy. STAT Plus (8 May 2017). https://www.statnews.com/2017/05/08/patient-dies-kite-car-t-therapy/
- M. B. Yaffe, N. R. Gough, 2012: Signaling breakthroughs of the year. Sci. Signal. 6, eg1 (2013). PubMed https://www.ncbi.nlm.nih.gov/pubmed/23281367
- J. C. Fitzgerald, S. L. Weiss, S. L. Maude, D. M. Barrett, S. F. Lacey, J. J. Melenhorst, P. Shaw, R. A. Berg, C. H. June, D. L. Porter, N. V. Frey, S. A. Grupp, D. T. Teachey, Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit. Care Med. 45, e124-e131 (2017). PubMed https://www.ncbi.nlm.nih.gov/pubmed/27632680
- S. Mohammed, S. Sukumaran, P. Bajgain, N. Watanabe, H. E. Heslop, C. M. Rooney, M. K. Brenner, W. E. Fisher, A. M. Leen, J. F. Vera, Improving chimeric antigen receptor-modified T cell function by reversing the immunosuppressive tumor microenvironment of pancreatic cancer. Mol. Ther.25, 249–258 (2017). https://www.ncbi.nlm.nih.gov/pubmed/28129119
- C. L. Mackall, Genetically engineered T cells for cancer. AACR Annual Meeting 2017. http://www.abstractsonline.com/pp8/#!/4292/presentation/161
- C.-Y. Wu, K. T. Roybal, E. M. Puchner, J. Onuffer, W. A. Lim, Remote control of therapeutic T cells through a small molecule–gated chimeric receptor. Science350, aab4077(2015). https://www.ncbi.nlm.nih.gov/pubmed/
- S. Gill, CAR T cells engage in anticancer martial arts. Sci. Transl. Med. 9, eeal4996 (2017). https://www.ncbi.nlm.nih.gov/pubmed/28100838
- S. Chevrier, J. H. Levine, V. R. T. Zanotelli, K. Silina, D. Schulz, M. Bacac, C. H. Ries, L. Ailles, M. A. S. Jewett, H. Moch, M. van den Broek, C. Beisel, M. B. Stadler, C. Gedye, B. Reis, D. Pe’er, B. Bodenmiller, An immune atlas of clear cell renal cell carcinoma. Cell 169, 736-749 (2017). https://www.ncbi.nlm.nih.gov/pubmed/28475899
- Y. Lavin, S. Kobayashi, A. Leader, E.-a. D. Amir, N. Elefant, C. Bigenwald, R. Remark, R. Sweeney, C. D. Becker, J. H. Levine, K. Meinhof, A. Chow, S. Kim-Shulze, A. Wolf, C. Medaglia, H. Li, J. A. Rytlewski, R. O. Emerson, A. Solovyov, B. D. Greenbaum, C. Sanders, M. Vignali, M. B. Beasley, R. Flores, S. Gnjatic, D. Pe’er, A. Rahman, I. Amit, M. Merad, Innate immune landscape in early lung adenocarcinoma by paired single-cell analysis. Cell 169, 750-765 (2017). https://www.ncbi.nlm.nih.gov/pubmed/28475900
- C. A. Jacobson, J. Ritz, Time to put the CAR-T before the horse. Blood 118, 4761-4762 (2011). https://www.ncbi.nlm.nih.gov/pubmed/22053170
Cite as: N. R. Gough, Fighting Cancer with the Immune System: Lessons from CAR T Cell Therapy. BioSerendipity (15 May 2017). https://www.bioserendipity.com/lessons-from-car-t-cell-therapy/
Also of interest