Toxicity to healthy cells limits the usefulness of conventional chemotherapeutic agents. These drugs kill rapidly dividing cells of all types, not just those in a tumor. In addition to dividing rapidly, some cancer cells establish metastases that have a different stiffness than the surrounding tissue. This mechanical property of a cellular microenvironment is referred to as the mechano-niche. For example, breast cancer cells establish metastases at stiffer sites and will even secrete a protein that crosslinks proteins in the extracellular matrix to make a site stiffer.
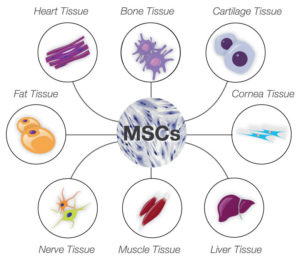
Mesenchymal stem cells (MSCs) are also mechanoresponsive cells and activate different gene expression profiles in response to the stiffness of their environment. Mechanical and chemical signals stimulate MSCs to form different kinds of cells, including bone, muscle, and neurons (Figure 1). Thus, many studies and clinical trials are underway to use MSCs in regenerative medicine.
In animal models of cancer, injected MSCs accumulate in tumors, making MSCs a potentially viable way to deliver anticancer drugs specifically to tumor cells. However, not all of the injected MSCs migrate to the tumor, so the potential for off-target effects is possible. Liu and colleagues took advantage of the mechanoresponsive gene expression of MSCs. They engineered MSCs to express the gene encoding an enzyme that activates an anticancer prodrug when the cells encountered a stiff environment, like that in a breast tumor. The enzyme is cytosine deaminase, which converts the prodrug 5-fluorocytosine (5-FC) into 5-fluorouracil (5-FU), an inhibitor of DNA replication. All cells have some cytosine deaminase. However, when cultured on a stiff substrate, the engineered MSCs produce much more of this enzyme, efficiently convert 5-FC into 5-FU, and eventually are killed by the 5-FU that is produced.
The authors found that the engineered MSCs triggered breast cancer cell death only when cocultured on a stiff surface and that breast cancer cell death occurred before MSC death. Thus, the engineered MSCs produced sufficient amounts of the toxic 5-FU to kill cells that were cocultured with them, but this toxic effect of the MSCs was limited to conditions of the proper stiffness. More importantly, when tested in two different mouse models of metastatic breast cancer, the engineered MSCs not only accumulated at the site of the tumors in the lungs, but also reduced tumor size, prolonged survival, and did not cause damage to healthy regions of the lung. In contrast, MSCs that expressed cytosine deaminase constitutively, without the mechanoresponsive control, also reduced tumor size and prolonged survival, but caused tissue damage to healthy areas of the lung. Thus, the inclusion of the mechanoresponsive control was key to ensuring targeted conversion of the prodrug and toxicity to the areas with the tumors.
This study illustrates at least two important concepts:
- Drug development can involve devising new ways to increase the usefulness of existing drugs, in this case by limiting toxicity to healthy cells.
- Integrating knowledge of cell regulatory pathways with synthetic biology can produce biologic agents (here, cell-based therapies) with context-specific activities of therapeutic benefit.
Related Reading
L. Liu, S. X. Zhang, W. Liao, H. P. Farhoodi, C. W. Wong, C. C. Chen, A. I. Ségaliny, J. V> Chacko, L. P. Nguyen, M. Lu, G. Polovin, E. J. Pone, T. L. Downing, D. A. Lawson, M. A. Digman, W. Zhao, Mechanoresponsive stem cells to target cancer metastases through biophysical cues. Sci Transl Med. 9, eaan2966 (2017). https://www.ncbi.nlm.nih.gov/pubmed/28747514
A. De Becker, I. V. Riet, Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J. Stem Cells 8, 73–87 (2016). https://www.ncbi.nlm.nih.gov/pubmed/27022438
Cite as: N. R. Gough, Leveraging Cellular Mechanical Signaling to Improve Drug Specificity. BioSerendipity (2 August 2017) https://www.bioserendipity.com/2017/08/02/leveraging-cellular-mechanical-signaling-to-improve-drug-specificity/.