A main goal of current research and clinical trials is to expand the number of patients that benefit from immune checkpoint inhibitors. Thus, it is perhaps not surprising that combination therapies are the future for these immunotherapies in treating cancer. There are many clinical trials that combine an immunotherapy with conventional chemotherapy (drugs that kill rapidly dividing cells) or with targeted therapy (drugs that target aberrant proteins or pathways in the tumor). Some trials have tested combining two immune checkpoint inhibitors (a PD-L1/PD-1 inhibitor with a CTLA-4 inhibitor). Given that the immune checkpoint inhibitors do not specifically reactivate only the immune cells that recognize and target the cancer cells, combining multiple immune checkpoint inhibitors can result in increased adverse effects.
There are at least two clinical trials testing engineered T cells with chimeric antigen receptors (CAR T cells) that are also engineered to produce a antibodies that inhibit the PD-L1/PD-1 immune checkpoint. The primary outcome of one of the trials is testing the safety of this cell-based immunotherapy in patients with advanced mesothelin-positive solid cancers. The other trial is testing safety as a primary outcome in patients with glioblastoma receiving CAR T cells that are also producing antibodies against PD-L1 and PD-1 (Figure 1).
Expanding the usefulness of immune checkpoint inhibitors will require multiple strategies. because there are several ways that cancers can evade immune recognition. Some cancers use combinations of these mechanisms to avoid destruction by the immune system and different mechanisms may apply to various metastatic sites or even to heterogeneous tumors at a single site. Furthermore, as cancers evolve to survive and proliferate in the patients as they undergo therapy, it is likely that treatment strategies will have to evolve for the patient as well.

Some cancers are “cold”, meaning that there are no immune cells within the tumor. Somehow, the tumor blocks the immune cells from penetrating (Figure 2). Others are an immune “desert,” in that the immune cells do not even come close to the tumor. For these cancer patients, reactivating the tumor-targeted T cells will not be effective; the reactivated cytotoxic T cells cannot get to the tumor.
Other tumors release signals that alter cells of the innate immune system to produce an immunosuppressive condition. Some cancers behave as if they are chronic injuries and are associated with inflammation. Although these cancers are “hot” in terms of infiltrated immune cells, this chronic inflammatory response can cause immune cell “exhaustion” such that the cytotoxic immune cells no longer function.
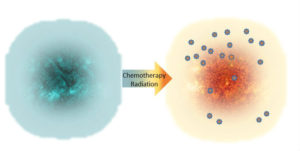
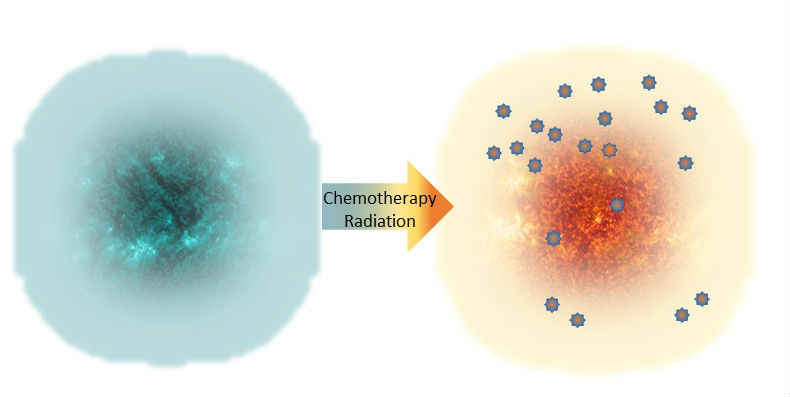
Despite this complexity, there are options under investigation that may overcome one or more of these evasion strategies. For example, pretreatment with a chemotherapy agent or targeted radiation may initiate sufficient tumor destruction and damage so that an immunologically “cold” tumor is converted to one that is “hot” and becomes infiltrated by the immune system (Figure 3). Additional therapeutic strategies may overcome exhaustion, enabling the immune system to maintain the anticancer response.
The next in the series highlights what can be learned from the patients who are immune checkpoint inhibitor “super” responders and studies that combine other immune modulators with immune checkpoint inhibitors.
Related Reading
PD-1 antibody expressing chimeric antigen receptor T cells for mesothelin positive advanced malignancies. ClinicalTrials.gov Identifier: NCT03030001
4SCAR-IgT cells expressing PD-L1 and PD1 antibodies against glioblastoma multiform. ClinicalTrials.gov Identifier: NCT03170141
N. Shaverdian, A. E. Lisberg, K. Bornazyan, D. Veruttipong, J. W. Goldman, S. C. Formenti, E. B. Garon, P. Lee, Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. (2017 May 24) DOI: 10.1016/S1470-2045(17)30380-7. PubMed
Cite as: N. R. Gough, Making Immune “Cold” Tumors Hot. BioSerendipity (31 May 2017). https://www.bioserendipity.com/2017/05/31/making-immune-cold-tumors-hot/