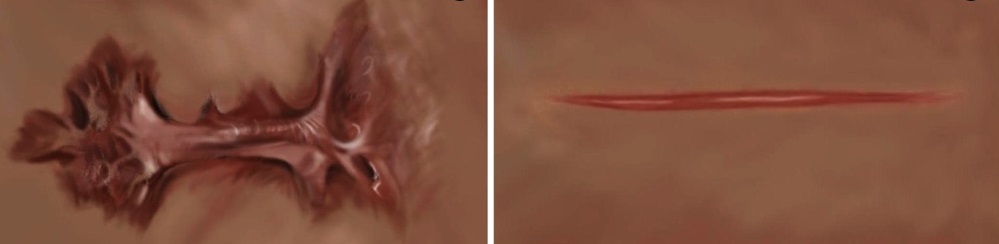
Scars are the body’s response to skin injury. Sometimes too much of the protein collagen is produced at the site of the injury, resulting in excessively large thick scars that can be disfiguring, uncomfortable, and compromise function by restricting mobility. These can either be hypertrophic scars, which are thickened but limited to the injured area, or keloid, which exceed the bounds of the injury (Figure 1). Effective intervention to prevent these types of scars requires the ability to detect the abnormal fibroblasts at the wounded region. Presently, this requires a biopsy of the scar and analysis by a pathologist. Yeo and colleagues show that a gold nanoparticle-based approach can detect the abnormal cells without requiring a biopsy, which could enable earlier detection and more effective intervention.
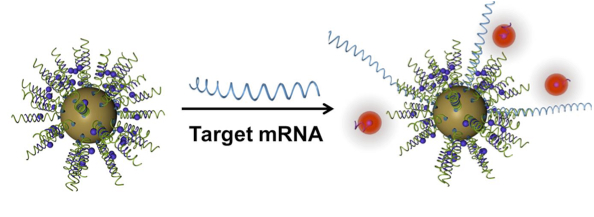
The authors used NanoFlares that recognize the expression of a gene encoding a growth factor that triggers the excessive production of collagen. Not to be confused with the bursts of heat in the atmosphere of the Sun, which are also called nanoflares, NanoFlares are composed of a gold nanoparticle to which a nucleic acid sequence is attached (Figure 2). The nucleic acid portion consists of 2 components–the recognition sequence specific for the transcript (mRNA) of the expressed gene and the reporter sequence that is coupled to a fluorescent molecule and binds the recognition sequence. The target mRNA competes with the reporter sequence for the recognition sequence and when the reporter sequence is released, it emits light. NanoFlares have been used to detect the expression of genes associated with cancer metastasis in isolated or cultured cells. Here, the NanoFlares are used as a topical reagent on the skin to detect the abnormal cells that stimulate excessive scar formation.
The abnormal cells that produce too much collagen are dermal fibroblasts that express too much the gene encoding TGF-β1 (transforming growth factor β1), which in turn stimulates too much expression of the gene encoding CTGF (connective tissue growth factor). The result is too much collagen deposition. Yeo and colleagues designed the NanoFlare to detect transcripts of CTGF. First, they used cultured cells from injured skin, regions near injured skin, and noninjured skin to show that the CTGF-targeted NanoFlares produced a brighter signal in hypertrophic scar fibroblasts and keloid scar fibroblasts, as well as the fibroblasts adjacent to the scar, than in normal dermis fibroblasts. These different fibroblast populations represent cells with different amounts of CTGF transcripts, suggesting that the NanoFlares are sensitive enough to detect changes in CTGF expression.
Such a sensitive fluorescent reagent may be useful in screening drugs that could reduce excessive scarring. Using hypertrophic scar fibroblasts in culture, the authors showed that the CTGF-targeted NanoFlares detected changes in the abundance of CTGF transcripts in response to either agonists that activated the TGF-β pathway or antagonists of this pathway. The authors even discovered two compounds that may be useful in treating excessive scar formation: RepSox (an inhibitor of the TGF-β receptor ALK5) and thiazovivin (an inhibitor of Rho kinase).
To determine if the CTGF-targeted NanoFlares could detect differences in skin tissue, the authors used a human skin tissue model and animal models. The authors injected either normal dermal fibroblasts or hypertrophic scar fibroblasts on the dermal side of the human skin samples. On the epithelial side (the side that would be exposed to the outside), the authors applied the NanoFlares in a thick clear skin moisturizer (Aquaphor). The NanoFlares produced a strong signal in the skin injected with the hypertrophic scar fibroblasts. The NanoFlares also produced a strong signal when applied to mouse skin at sites that had been injected with hypertrophic scar fibroblasts. In a rabbit wound model, the CTGF-targeted NanoFlares produced a stronger signal at healed wounds with hypertrophic scars than at unwounded skin. In wounds that were in the process of healing, the signal from the CTGF-targeted NanoFlares increased during the healing process and correlated with the formation of hypertrophic scars, indicating that these NanoFlares may have diagnostic potential for detecting the abnormal fibroblasts that cause excessive scarring.
Related nanoparticles that have a nucleic acid sequence that stimulates the destruction of the target mRNA, instead of a reporter RNA, are under investigation for cancer treatment and the treatment of psoriasis. Because the NanoFlares penetrated the skin effectively to reach the fibroblasts in the dermal region, it is possible that a similar strategy could be used to deliver nanoparticle-based therapeutics to reduce the signals that trigger the excessive scar formation. First, the NanoFlare would detect abnormal fibroblasts, then a preparation that includes a nanoparticle-based therapeutic could be applied and the NanoFlare used to monitor effectiveness.
Highlighted Article
D. C. Yeo, C. Wiraja, A. S. Paller, C. A. Mirkin, C. Xu, Abnormal scar identification with spherical-nucleic-acid technology. Nat. Biomed. Eng. 2, 227-238 (2018). Online Journal
Related Reading
Tiffany L. Halo, Kaylin M. McMahon, Nicholas L. Angeloni, Yilin Xu, Wei Wang, Alyssa B. Chinen, Dmitry Malin, Elena Strekalova, Vincent L. Cryns, Chonghui Cheng, Chad A. Mirkin, and C. Shad Thaxton, NanoFlares for the detection, isolation, and culture of live tumor cells from human blood. Proc. Natl. Acad. Sci. U.S.A. 111, 17104-17019 (2014). PubMed
Houshang Nemati, Mohammad-Hosein Ghahramani, Reza Faridi-Majidi. Babak Izadi, Gholamreza Bahrami, Seyed-Hamid Madani, Gholamreza Tavoosidana, Using siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation in psoriasis. J. Control. Release 268, 259-268 (2017). PubMed
Samuel A. Jensen, Emily S. Day, Caroline H. Ko, Lisa A. Hurley, Janina P. Luciano, Fotini M. Kouri, Timothy J. Merkel, Andrea J. Luthi, Pinal C. Patel, Joshua I. Cutler, Weston L. Daniel, Alexander W. Scott, Matthew W. Rotz, Thomas J. Meade, David A. Giljohann, Chad A. Mirkin, Alexander H. Stegh, Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci. Transl. Med. 5, 209ra152 (2013). PubMed
Cite as: N. R. Gough, Scar-Detecting Nanoparticles. BioSerendipity (9 May 2018) https://www.bioserendipity.com/scar-detecting-nanoparticles/