Secreted proteins or the parts of transmembrane proteins that are extracellular can be phosphorylated. DeRita and colleagues reported that the cytosolic tyrosine kinase c-SRC was one of several kinases released from prostate cancer cells in exosomes. Sánchez-Pozo and colleagues identified a cancer-relevant function for extracellular c-SRC: c-SRC functioned outside of cultured cells to regulate the activity of tissue inhibitor of metalloproteinases 2 (TIMP2).
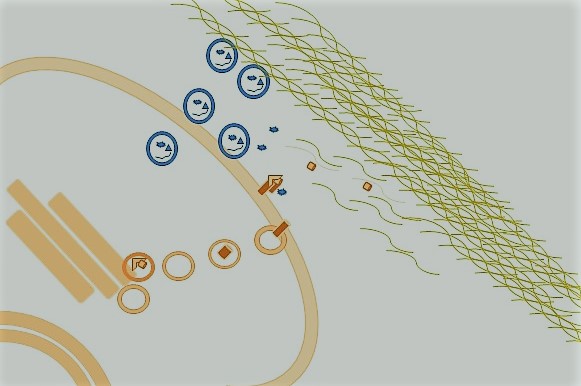
The study by Sánchez-Pozo and colleagues used cultured human cell lines and found that the secreted protein TIMP2 was phosphorylated on a specific tyrosine residue. TIMP2 is a dual-function protein. Along with another protein (membrane type 1-matrix metalloproteinase, MMP14), TIMP2 binds and activates the latent proenzyme form of MMP2 (Figure 1); TIMP-2 also inhibits the activity of the mature form of MMP2. MMP2 is an extracellular enzyme that degrades proteins found in the extracellular matrix, such as collagen, and thereby affects cell migration. MMP2 also contributes to vascularization, the development of lymph vessels, and cleavage-mediated activation or release of growth-regulating factors and signaling molecules. MMP2 abundance is increased in some cancers. Thus, understanding the regulation of MMP2 activity is clinically important.

The authors blocked the release of TIMP2 into the medium using the chemical brefeldin A, which prevents movement of proteins from the endoplasmic reticulum to the Golgi and thus secretion by this pathway, and found that this prevented the tyrosine phosphorylation of the protein. Brefeldin A did not prevent c-SRC release into the medium, indicating that c-SRC was released from cells through a pathway distinct from that used to release TIMP2, which would be consistent with exosome-mediated release (Figure 2). Addition of a function-blocking antibody against c-SRC to the cell culture medium blocked the phosphorylation of TIMP2.
The phosphorylation of TIMP2 on tyrosine 90 was necessary for TIMP2 to interact with the pro (nonactivated)-form of MMP2. A mutation that mimicked this phosphorylation resulted in TIMP2 that had enhanced ability to inhibit active MMP2. A mutation that blocked phosphorylation at this site prevented the interaction and activation of proMMP2 but did not impair inhibition of active mature MMP2. Thus, TIMP2 phosphorylation appears required for the interaction between TIMP2 and proMMP2 and enhances the inhibitory action of TIMP2 for mature MMP2. Reconciling these findings with the existing structural data, which were acquired from proteins without phosphorylation, remains an area for investigation.
Of interest to cell biologists, biochemists, and cancer researchers is the concept that phosphorylation-mediated regulation of proteins may occur after proteins have been secreted. Thinking of cytosolic kinases as only active inside cells may be too narrow a view. A remaining question relates to the conditions enabling kinases that are released from cells to be active. These studies add a new level of complexity to our understanding of protein regulation by phosphorylation.
References
R. M. DeRita, B. Zerlank, A. Singh, H. Lu, R. V. Iozzo, J. L. Benovic, L. R. Languino, c‐Src, Insulin‐Like Growth Factor I Receptor, G‐Protein‐Coupled Receptor Kinases and Focal Adhesion Kinase are Enriched Into Prostate Cancer Cell Exosomes. J. Cell. Biochem. 118, 66-73 (2017). 10.1002/jcb.25611 PubMed
Proteins
MT1-MMP (also known as MMP14) NCBI Gene UniProt
Cite as: N. R. Gough, SRC on the Outside. BioSerendipity (26 March 2018) https://www.bioserendipity.com/src-on-the-outside/