This commentary on the power of proteomics highlights research showing how proteomics can advance the diagnosis of atherosclerosis, a type of vascular disease. Langley and colleagues use mass spectrometric analysis to define a 4-protein signature that predicts patients with atherosclerosis who are at risk for a vascular event, such as stroke or heart attack.This study illustrate the potential benefit of using proteomic data, not just genomic and transcriptomic data, to establish clinically useful diagnostic tools for assessing risk and disease progression.
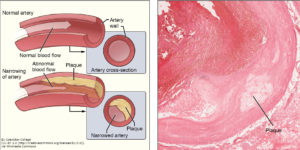
For the third validation of the candidate biomarkers, the authors switched to a different system. They used mass spectrometry to compare the secreted proteins (secretome) of control and lipid-loaded human vascular smooth muscle cells, which are part of atherosclerotic plaques and contribute to the inflammation associated with these lesions. Approximately half of the proteins enriched in the secretome of the cultured lipid-loaded smooth muscle cells were the same as those identified in the atherosclerotic plaque samples, including the CTSB and the related enzyme cathepsin D (CTSD), FN1, and calprotectin. These results are consistent with a role for these cells in the formation of atherosclerotic lesions. In addition to the proteins identified through the analysis of the carotid artery samples, the smooth muscle cell analysis identified galectin-3-binding protein (LGALS3BP, a macrophage scavenger receptor protein) as increased. LGALS3BP was released from human aortic tissue exposed to MMP9, one of the abundant enzymes in the extracellular matrix of plaques from symptomatic patient samples.
From the combination of the three approaches, the authors selected FN1, calprotectin, LGALS3BP, MMP9, and CTSD (instead of CTSB for technical reasons), and CHI3L1 as the biomarker signature to test in two cardiovascular disease and atherosclerosis studies. From blood samples from the patients in the studies, they computed the statistical association of each of the biomarkers with disease progression in patients in the Bruneck and SAPHIR studies. In the Bruneck study, the patients were older and stroke was a more common cardiovascular event than heart attack; whereas, in the SAPHIR study, the patients were ~14 years younger on average and heart attack was more common than stroke. The amount of the individual biomarkers—FN1, calprotectin, LGALS3BP, MMP9, and CTSD—in blood samples significantly correlated with progression to advanced atherosclerosis and incidence of cardiovascular disease events (stroke, heart attack, or death by a vascular event). CHI3L1 was only a predictive indicator in the Bruneck patients, consistent with its specific involvement in stroke.
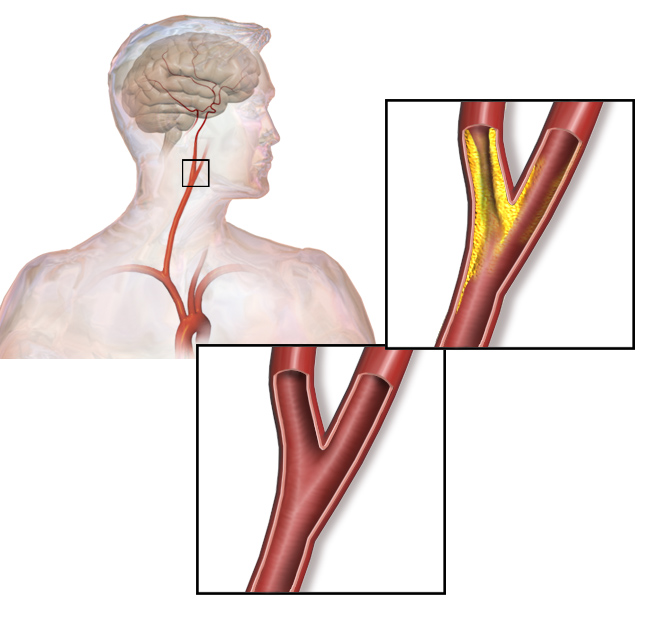
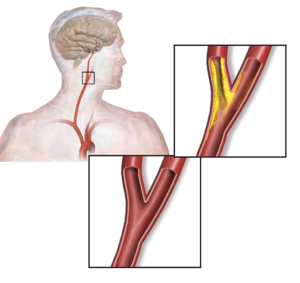
The authors propose that using the biomarker signature comprised of calprotectin, LGALS3BP, MMP9, and CTSD may (i) aid clinicians in assessing patient risk, especially for those with carotid artery stenosis (Figure 2), (ii) inform clinicians about the origin of a stroke, and (iii) be a diagnostic tool for assessing cardiovascular disease before markers of tissue damage become detectable. Of the proteins identified as part of this signature, only LGALS3BP (also known as MAC-2) has not been associated with atherosclerosis previously. Thus, this study did not provide many new candidates for understanding the mechanism of disease progression or prevention of this vascular disease.
Highlighted Article
S. R. Langley, K. Willeit, A. Didangelos, L. P. Matic. P. Skroblin, J. Barallobre–Barreiro, M. Lengquist, G. Rungger, A. Kapustin, L. Kedenko, C. Molenaar, R. Lu, T. Barwari, G. Suna, X. Yin, B. Iglseder, B. Paulweber, P. Willeit, J. Shalhoub, G. Pasterkamp, A. H. Davies, C. Monaco, U. Hedin, C. M. Shanahan, J. Willeit, S. Kiechl, M. Mayr, Extracellular matrix proteomics identifies molecular signature of symptomatic carotid plaques. J. Clin. Invest. 127, 1546-1560 (2017). PubMed
Cite as: N. R. Gough, The Power of Proteomics in Vascular Disease. BioSerendipity (19 June 2017). https://www.bioserendipity.com/2017/06/19/the-power-of-proteomics-in-vascular-disease/