Neuropathic pain can be caused by an excessive nerve response to non-harmful (innocuous) touch. Dhandapani and colleagues combined a molecule that binds to the neurons and a light-activated phototoxic chemical to create a treatment that uses light (specifically near infrared light) to alleviate neuropathic pain in a mouse model. To develop this treatment, the authors first had to determine which neurons were responsible for mediating the aberrant response to innocuous touch. They used genetic engineering techniques in mice to identify the neurons to target.
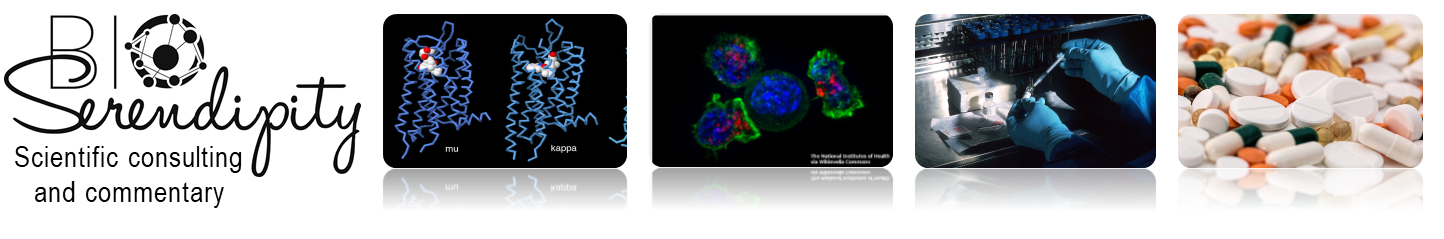
Sensory neurons with the receptor TrkB are thought to be involved in neuropathic pain. Therefore, the authors genetically engineered mice so that the TrkB-positive neurons also expressed a protein that turned only these neurons and their processes red, making them visible with a microscope. The authors traced these neurons and their processes and found that they extended into the skin at hair follicles and mechanical sensory structures called Meissner corpuscles (Figure 1). The neurons at the hair follicles are called D-hairs, and those at Meissner corpuscles are called rapidly adapting Aβ mechanoreceptors (RAMs). Staining the mouse ganglia for other neuronal markers showed that TrkB-positive nerves were large low threshold A-fiber mechanoreceptive nerves (LTMRs), not C-fiber mechanoreceptive nerves or nociceptive (pain-responsive) nerves. Human skin and nerve ganglia had a similar profile and innervation pattern (hair follicle and Meissner corpuscles) for TrkB neurons. The human TrkB neurons were identified by staining tissue samples with antibodies.

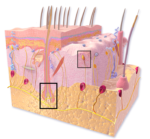
One of the genetic engineering techniques was optogenetics, which introduces a protein (called channelrhodopsin-2, ChR2) that activates neurons in response to light of a specific wavelength. By comparing the neuronal responses to light (473 nm blue) and mechanical stimulation (touch) in skin tissue from mice expressing ChR2 in TrkB-positive neurons, the authors found that light activated 2 of the 6 types of neurons that were also activated by mechanical stimulation (Figure 2). The two types of neurons were D-hair (also called Aδ-LTMR) and RAM (also called RA Aβ-LTMR). Using another genetic engineered mouse model in which these neurons were selectively eliminated in adult mice, the authors showed that the mice without most of their D-hair and RAM neurons responded normally to heat, cold, pinpricks, or relatively strong touch. However, these mice were specifically non-responsive to light touch.
Having established which neurons responded to light touch, the authors showed that these neurons also contributed to hypersensitive responses in mice with spared nerve injury (SNI, a neuropathic pain model) but not the hypersensitive responses following acute inflammation with an injected irritating chemical. When mice engineered with ChR2 in the TrkB-positive neurons were subjected to SNI, light stimulated behaviors (paw lifting and licking) associated with discomfort in the area exposed to the light.
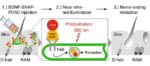
TrkB is a neurotrophin receptor that selectively binds the ligand brain-derived neurotrophic factor (BDNF). Coupling BDNF to a phototoxic chemical, which is converted to a toxic form by near infrared light (680 nm red), produced a light-activated ligand (BDNFSNAP-IR700) that selectively bound to TrkB-positive cells. When tested on a non-neuronal cell line in culture, BDNFSNAP-IR700 and near infrared light killed only TrkB-positive cells. Using 3 mouse models of neuropathic pain (the SNI model, a diabetic neuropathy model, and chemotherapy-induced neuropathy model), the authors showed that injection of BDNFSNAP-IR700 into the affected paw and then exposure to near infrared light reduced behavorial responses associated with discomfort in response to innocuous touch. The reduction in pain lasted more than 2 weeks, and efficacy of this photoablation therapy depended on both the concentration of BDNFSNAP-IR700 injected and the intensity of the light applied. Although the combination of BDNFSNAP-IR700 and near infrared light caused death of cells in culture, this combination caused only a reduction in the nerve endings in the skin, not death of the nerves, in the mice (Figure 3).
Unfortunately, TrkB is also present on cells positive for the protein CD34, which is a marker of mast cells of the immune system and progenitor cells that form epithelial cells and endothelial cells. The number of CD34-positive cells was reduced in response to the BDNF-targeted photoablation treatment, suggesting that this photoablation therapy was toxic to these cells and caused their death. Although the CD34 cells were not responsible for the reduction in TrkB-positive neuronal activity and thus the reduction in pain responses, toxicity toward these cells could cause unwanted side effects.
This study shows two different uses of lasers in research: The blue light laser was used to activate neurons with light through optogenetics and the red light laser was used to activate the phototoxic chemical to trigger retraction of the nerve endings that were causing the neuropathic pain. In pain research, this study provides strong evidence for the involvement of D-hairs and RAMs (at least in mice) in mediating some types of neuropathic pain. The genetically engineered mice will be valuable in dissecting neuronal pain and sensory circuits. Finally, this study provides an example of how laser ablation (also called photoablation) therapy can be directed to specific cells by targeting the cells with a specific molecule coupled to a light-activated phototoxic chemical.
Highlighted Article
R. Dhandapani, C. M. Arokiaraj, F. J. Taberner, P. Pacifico, S. Raja, L. Nocchi, C. Portulano, F. Franciosa, M. Maffei, A. F. Hussain, F. de Castro Reis, L. Reymond, E. Perlas, S. Garcovich, S. Barth, K. Johnsson, S. G. Lechner, P. A. Heppenstall, Control fo mechanical pain hypersensitivity in mice through ligand-targeted photoablation of TrkB-positive sensory neurons. Nat. Comm. 9, 1640, DOI: 10.1038/s41467-018-04049-3. PubMed
Cite as: N. R. Gough, Zapping Chronic Pain with Red Light. BioSerendipity (26 April 2018). https://www.bioserendipity.com/zapping-chronic-pain-with-red-light/